
Contact
Email: bentley@umd.edu
Call: (301) 405-4321
William Bentley
Distinguished University Professor
Bentley Group
Contact
Email: bentley@umd.edu
Call: (301) 405-4321
Education
- Ph.D., Chemical Engineering, University of Colorado, 1989
- M.Eng., Chemical Engineering, Cornell University, 1983
- B.S., Chemical Engineering, Cornell University, 1982
Profile
Dr. William E. Bentley develops and uses molecular tools to engineer cells for enhanced function (synthetic biology) and to open “communication” pathways for innovative device design and fabrication (bioelectronics). His laboratory has engineered cells and small consortia of cells to execute advanced functions such as detecting and killing pathogens. His lab has also adapted natural bacterial signaling pathways to build components and systems that enable bidirectional communication between devices and biological systems.
CURRENT RESEARCH
Metabolic and Biomolecular Engineering – Quorum Sensing and Cell Networks
The Bentley Lab creates and uses molecular tools to understand the regulation of genetic circuits during applied stresses, and to gain near real-time information on the dynamics of metabolites, genes, proteins, and protein assemblies in targeted circuits. They use systems biology and synthetic biology approaches to alter the intracellular environment to improve cellular processes.
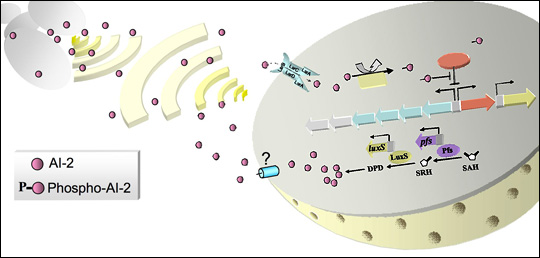 Many projects in the lab involve “quorum sensing” (QS), a signal transduction pathway of cell-to-cell communication about population levels that regulates cell behavior and enables individual bacteria to act with multicellularity. The Bentley Lab was the first group to use QS signaling to develop a means to control bacterial subpopulations and sort quantized quorums (Servinsky et al. 2015, ISME Journal). More recently, they exploited these signaling pathways that control individual and groups of cells to engineer bacteria to seek out and kill pathogens in the GI tract of mice (Hwang et al. 2017, Nature Comm). Here, the cells themselves are functioning products of synthetic biology, as they recognize, compute, actuate, and deliver -- all in a programmed manner.
Many projects in the lab involve “quorum sensing” (QS), a signal transduction pathway of cell-to-cell communication about population levels that regulates cell behavior and enables individual bacteria to act with multicellularity. The Bentley Lab was the first group to use QS signaling to develop a means to control bacterial subpopulations and sort quantized quorums (Servinsky et al. 2015, ISME Journal). More recently, they exploited these signaling pathways that control individual and groups of cells to engineer bacteria to seek out and kill pathogens in the GI tract of mice (Hwang et al. 2017, Nature Comm). Here, the cells themselves are functioning products of synthetic biology, as they recognize, compute, actuate, and deliver -- all in a programmed manner.
Biofabrication Engineering of Biological Signaling - bBIOS
The Bentley Lab is engaged in a multidisciplinary effort to create systems that serve to bridge the communication gap between biological systems and electronic microfabricated devices. Biology “communicates” via small molecule signaling (e.g. QS above) and ion flow, but devices are programmed with electrons, leading to a problem of translation. Dr. Bentley’s group employs the biopolymer chitosan as a “smart” stimuli-responsive interface. 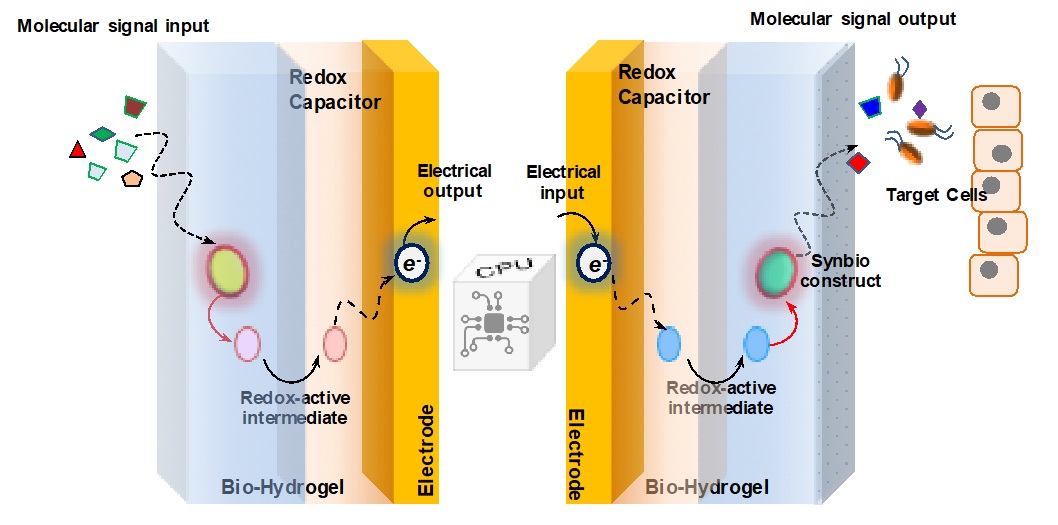
For example, the group developed a means to electronically assemble and control the activity of a two-enzyme biosynthetic pathway that leads to a bacterial QS signal molecule (Gordonov et al. 2014, Nature Nanotech). This showed for the first time that one can electrically connect to and control biological behavior. A signal molecule generated and controlled on-chip affected behavioral phenotype at the population level,
More recently, they demonstrated for the first time direct electrical actuation of gene expression in bacteria. They exploited redox signaling to actuate in a reversible manner expression of several genes in E. coli, including those that synthesize a native QS signal molecule. This molecule, in turn, actuated gene expression in neighboring cells. Hence, electrically programmed cells were made to stimulate signaling via native biological means. (Tshirhart et al. 2017, Nature Comm). For more information, visit the Maryland Biochip Collaborative website.
The Bentley group anticipates developing new tools for deciphering the presence of pathogens and for understanding and treatment of metabolic diseases, cancer, and hemorrhagic shock.