
Contact
Email: yuxingli@umd.edu
Call: (240) 314-6332
Yuxing Li
Professor
Yuxing Li Group
Contact
Email: yuxingli@umd.edu
Call: (240) 314-6332
Education
- Postdoctoral Research, Viral Immunology, Vaccine Research Center, NIAID/NIH, 2008
- Ph.D., Genetics, Iowa State University, 1996
- M.S., Microbiology, Sichuan Industrial Institute of Antibiotics, The State Food and Drug Administration (SFDA), 1992
- B.S., Genetics, Sichuan University, China, 1989
Profile
Dr. Yuxing Li’s lab studies how B cells, a critical part of the immune system, respond to viral infection, and applies these findings toward antibody discovery and the development of vaccines and therapeutics to treat viral infections.
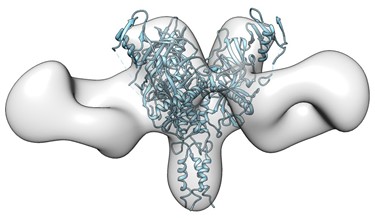
Dr. Li’s work has focused on defining broadly neutralizing antibody responses elicited by HIV-1 envelope glycoproteins during natural infections and in animal models. These findings contributed substantially to the in-depth understanding of HIV broadly neutralizing antibody response and the subsequent discovery of broadly neutralizing monoclonal antibodies targeting the HIV envelope glycoprotein receptor binding site, and have important implications for vaccine and immunotherapeutics development.
CURRENT RESEARCH
Development of B cell response targeting HIV-1 envelope glycoproteins (Env)
Dr Li’s work is focused on finding novel immunization regimens to better elicit broadly neutralizing antibodies. Dr. Li’s laboratory aims to better understand the mechanism underlying protective immunity and contribute to the development of a broadly effective HIV-1 vaccine.
Antigen-specific multi-color single B cell sorting and monoclonal antibody cloning
The Li lab is using rational design of HIV-1 (Env) immunogens and immunization regimens to analyze B cell and antibody responses with cutting-edge techniques. The Li lab is working to better define the B cell response to experimental HIV-1 vaccines toward discovering novel antibodies and gain insight into viral infection and vaccination.
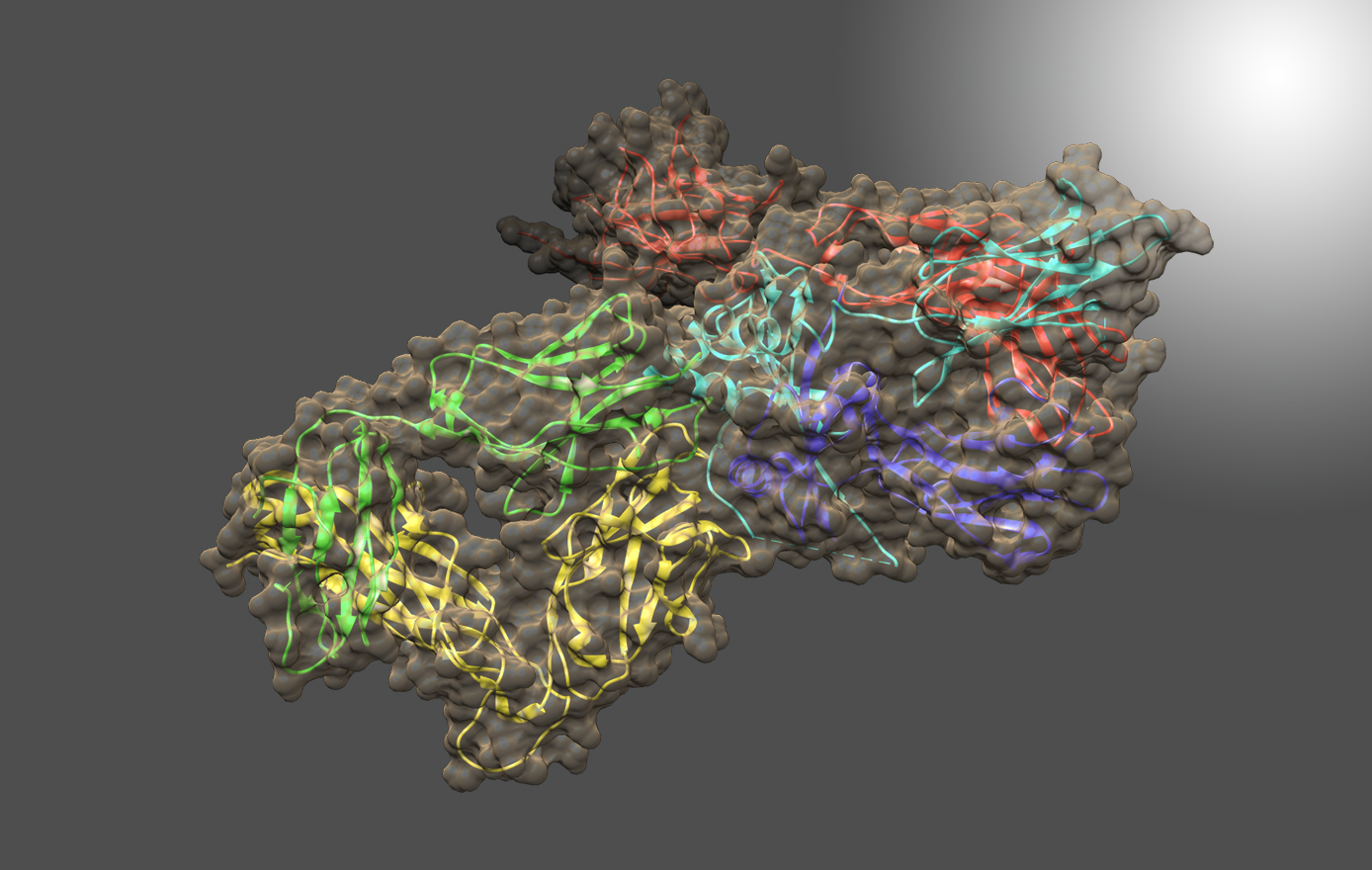
Development of novel anti-viral immunotherapeutics by structure-based antibody engineering
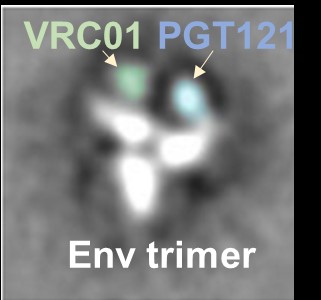
Recently, Dr. Li’s laboratory successfully created a bi-specific antibody that combined functional epitope-binding moieties from two broadly neutralizing antibodies, VRC01 and PGT 121, which simultaneously binds two epitopes within one Env trimer and neutralizes >96% of circulating
HIV-1 isolates. Multi-specific antibodies developed based on this design demonstrated improved antiviral breadth and potency.
In close collaboration with Integrated Biotherapeutics, Inc., the Li lab successfully isolated a broadly neutralizing antibody, CA45, from an Ebola virus vaccine candidate, immunized, non-human primate animal and developed a pan-Ebolavirus therapeutic antibody cocktail for a pre-clinical trial in a non-human primate model. Therapeutic antibodies for Ebola virus infection were only protective against the Zaire Ebola strain but not other related filoviruses. The lab’s research focus is on understanding B cell responses to the filovirus glycoproteins to inform vaccine design to develop a broadly protective antibody response.