Contact
Email: john.marino@nist.gov
Call: (240) 314-6361
John Marino
NIST
Contact
Email: john.marino@nist.gov
Call: (240) 314-6361
Education
- Postdoctoral Fellowship, Geothe Universität, Frankfurt, Germany, 1995 – 1997
- Ph.D., Chemistry, Yale University, 1995
- A.B., Chemistry, Princeton University, 1989
Profile
Dr. John Marino’s research is focused on developing nuclear magnetic resonance (NMR) and other biophysical measurements to accurately and precisely define the conformational structure, stability, and dynamics of biomolecules and their interactions at a molecular level.
In addition to enabling fundamental insights into biomolecular structure and function, Dr. Marino’s work provides innovative, yet practical methods that can form the basis for a robust measurement infrastructure that supports biopharmaceutical development and regulation.
CURRENT RESEARCH
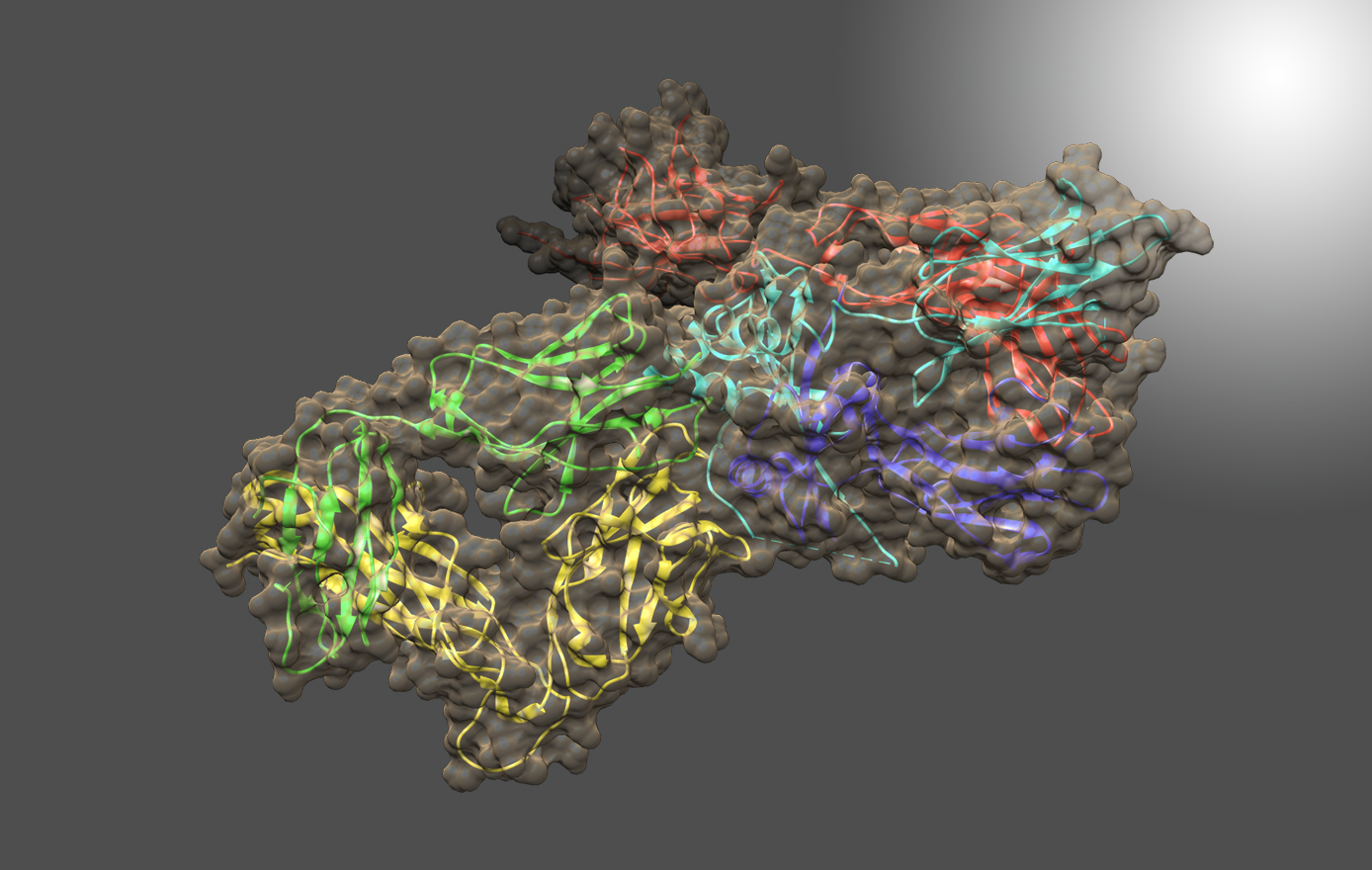
Biopharmaceuticals represent over 40% of the medicines in development today, making this industry a valuable and important sector of the US economy. A key focus is on the development of advanced techniques for accurate and precise characterization of higher-order-structure (HOS) in biotherapeutics, particularly monoclonal antibodies, which are sought by industry and regulators to establish consistency in drug manufacturing, detect process-related drug-product variations and compare biosimilars to innovator reference products.
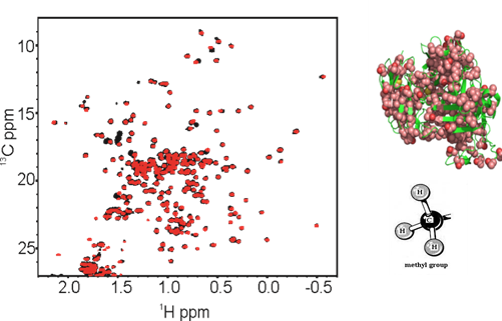
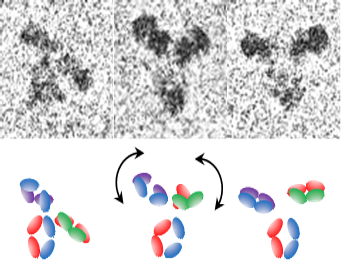
2D NMR for Biopharmaceutical Structure Assessment
Correct folding of biopharmaceuticals is critical for drug efficacy, with misfolding also impacting drug safety by eliciting unwanted immune and/or other off-target responses. High-resolution measurements of the structure(s) of protein therapeutics provide important tools for establishing consistency in drug manufacturing and detection of drug product changes resulting from modifications in the manufacturing process. NMR, a high-resolution and information-rich technique, is being applied as a tool for structural assessment of monoclonal antibodies (mAbs) biopharmaceuticals. This method is highly sensitive to molecular structure alterations that result from mutations, misfoldings, or contaminants. Experimental approaches are being developed to obtain structural 'fingerprints' of the bioactive form of protein therapeutics, as well as detection of variants, at atomic resolution. In parallel, computational alternatives (e.g., chemometrics and machine learning) to interactive analysis of spectral features are being explored.
Measurements of Biopharmaceuticals using Cryogenic Electron Microscopy (cryo-EM)
In biopharmaceutical applications, analysis of small, asymmetric molecules, which exhibit inherent flexibility, is required. Cryo-EM approaches are being explored that will enable high-resolution (better than 0.3 nm) structural characterization of flexible, protein-based drugs that are below a molecular weight limit of ~200 kDa. To address current limitations of low contrast and the challenges with alignment and averaging of small, flexible biomolecules, new strategies for bioengineering sample platforms, image analysis, and mathematical modeling are being pursued.