New Study Demonstrates Tools for Understanding the Mechanisms of Alzheimer's Disease
Fri, Jun 9, 2023
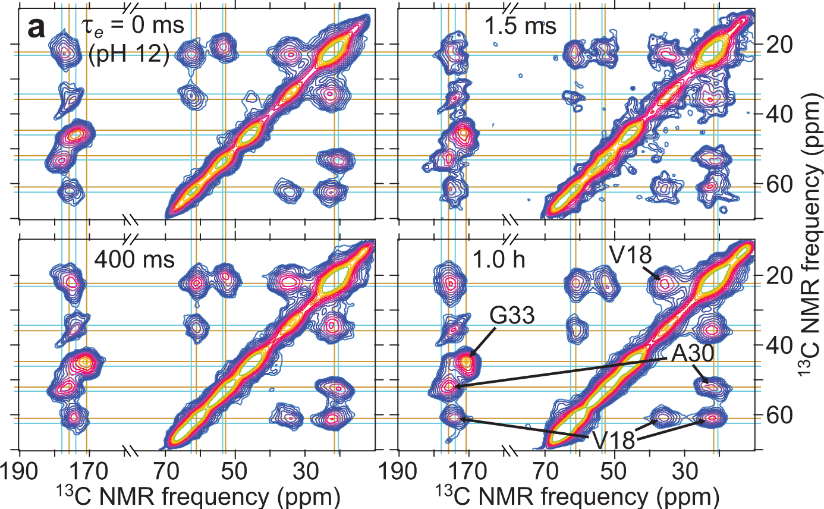
Amyloid-β peptide aggregation and fibril formation have been the subject of very active research in the field since they are suspected to be responsible for Alzheimer's disease (AD). However, many important molecular structural aspects at early stages of these processes have not been accessible by standard experimental approaches because early oligomers are short-lived and heterogeneous. In recent Nature Communications paper
(https://doi.org/10.1038/s41467-023-38494-6), published by Dr. Jaekyun Jeon, IBBR Research Assistant Professor and his collaborators at the National Institutes of Health, addressed the challenged on characterizing the early stages of fibril formation by applying two novel biophysical experimental approaches involving time-resolved solid-state Nuclear Magnetic Resonance (ssNMR) and light scattering. The techniques developed by Jeon and his colleagues provided the tools needed to gain important new insights into molecular structure in the early stages of this process, including measurement of secondary structure formation and molecular arrangements that depended on the sizes of oligomers.
