IBBR Researchers Awarded $2.1M from the NIH to Develop Novel Designer Proteases - “SMART Molecules” That Attack the Drivers of Cancer and Other Diseases
Tue, Sep 28, 2021
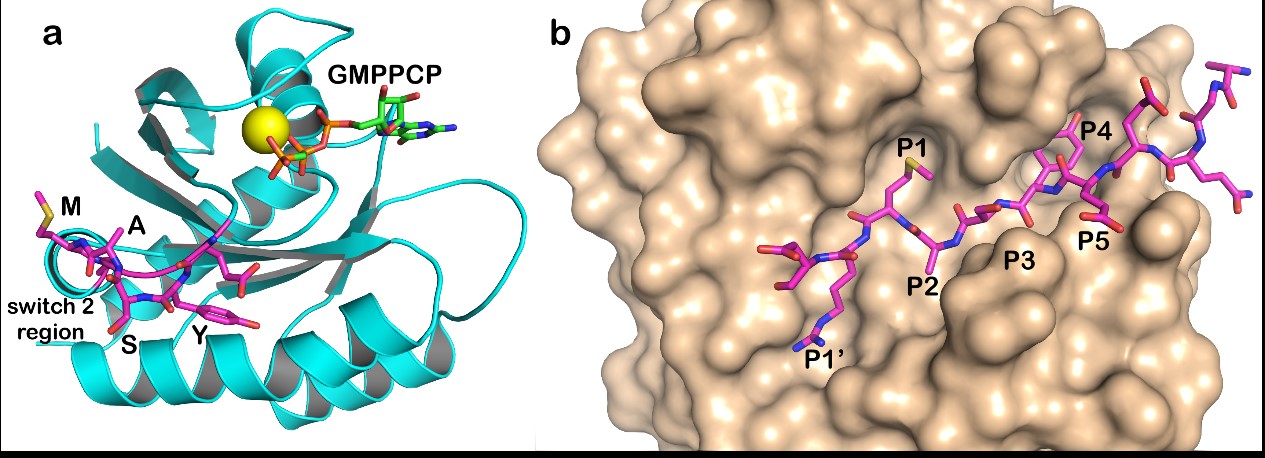
September 28, 2021 - Researchers at the Institute for Bioscience and Biotechnology Research (IBBR) and Potomac Affinity Proteins - a Maryland Biotech, were recently awarded $2.1 million by the National Institutes of Health (R01 GM141290) to develop novel designer proteases that attack drivers of cancer and other diseases. IBBR Fellows Drs. John Orban and Eric Toth and IBBR Fellow Emeritus Dr. Phil Bryan are the principal investigators on the award.
The project is part of a broader program whose aim is to design and engineer advanced protein-based therapeutics – so called ‘SMART molecules’ – that have the ability to ‘switch on’ to selectively act on a disease causing molecular target and then ‘switch off’ to revert to an inactive state. This mechanism of action combines potent activity with precise regulation to facilitate a system in which only the intended target is acted upon, while limiting drug side effects that normally come from actions on unintended molecular targets. Additional precision is built into the SMART molecule by making activity dependent on changes in the cellular environment unique to the disease state. Thus, the scientists of IBBR’s SMART therapeutics program are working to engineer “smart” assemblies of proteins that can dynamically respond to their environments and alter cellular signaling and responses, with the ultimate goal of combating disease.
IBBR’s first proof-of-principle SMART therapeutics project was the design of an enzyme that selectively destroys the cancer-promoting RAS protein (Figure 1). Mutations in one of the three RAS genes are involved in roughly a third of all human cancers, but therapeutic interventions have shown limited clinical efficacy. RAS-destroying proteases developed by Drs. Yingwei Chen, Biao Ruan, and Phil Bryan of Potomac Affinity Proteins became the proto-type SMART components. In many cancers, the disease is initiated and perpetuated by RAS being stuck in the active state (i.e. it is always “turned on”). The research team, that includes PIs from Potomac Affinity Proteins, University of Maryland, College Park, University of Maryland, Baltimore, NIST and USAMRIID has used their SMART molecule design paradigm to exploit this property of RAS. The “on” and “off” states are structurally and dynamically distinct from each other, allowing the team to incorporate preferential recognition of the “on” state into their design. As a result, the property of RAS that drives cancer, i.e. being stuck “on”, also drives its destruction by the RAS-specific protease SMART molecule system.
To date, the SMART therapeutics team has created a designer protease that selectively destroys active RAS in a test tube and in cell model systems1. Their next step will be to see how well their machine controls RAS signaling in cancer cell models. The team will then take the principles learned in the RAS study and apply them to other therapeutic targets. The project originated via an initiative by former IBBR Director Dr. Thomas Fuerst, who is a co-Investigator on the grant focusing on the cellular consequences of RAS-specific protease activity. Initial funding for the SMART protease research was provided through a seed grant and core-program support from the University of Maryland Strategic Partnership: MPowering the State, a program designed to leverage the strengths and missions of the University of Maryland, College Park and the University of Maryland, Baltimore.
The SMART molecule research team affiliations:
John Orban: Professor, UMCP Department of Chemistry and Biochemistry
Eric Toth: Assistant Research Professor, IBBR
Phil Bryan: Founder, Rockville-based Potomac Affinity Proteins, LLC
Thomas Fuerst: Professor, UMCP Department of Cell Biology and Molecular Genetics
References
Chen Y, Toth EA, Ruan B, Choi EJ, Simmerman R, Chen Y, He Y, Wang R, Godoy-Ruiz R, King H, Custer G, Travis Gallagher D, Rozak DA, Solomon M, Muro S, Weber DJ, Orban J, Fuerst TR, Bryan PN. 2021, Engineering subtilisin proteases that specifically degrade active RAS. Commun Biol. 2021 Mar 5;4(1):299. doi: 10.1038/s42003-021-01818-7.
