IBBR Researchers Collaborate with the FDA to evaluate wNMR for Biomanufacturing Automation
Tue, May 14, 2024
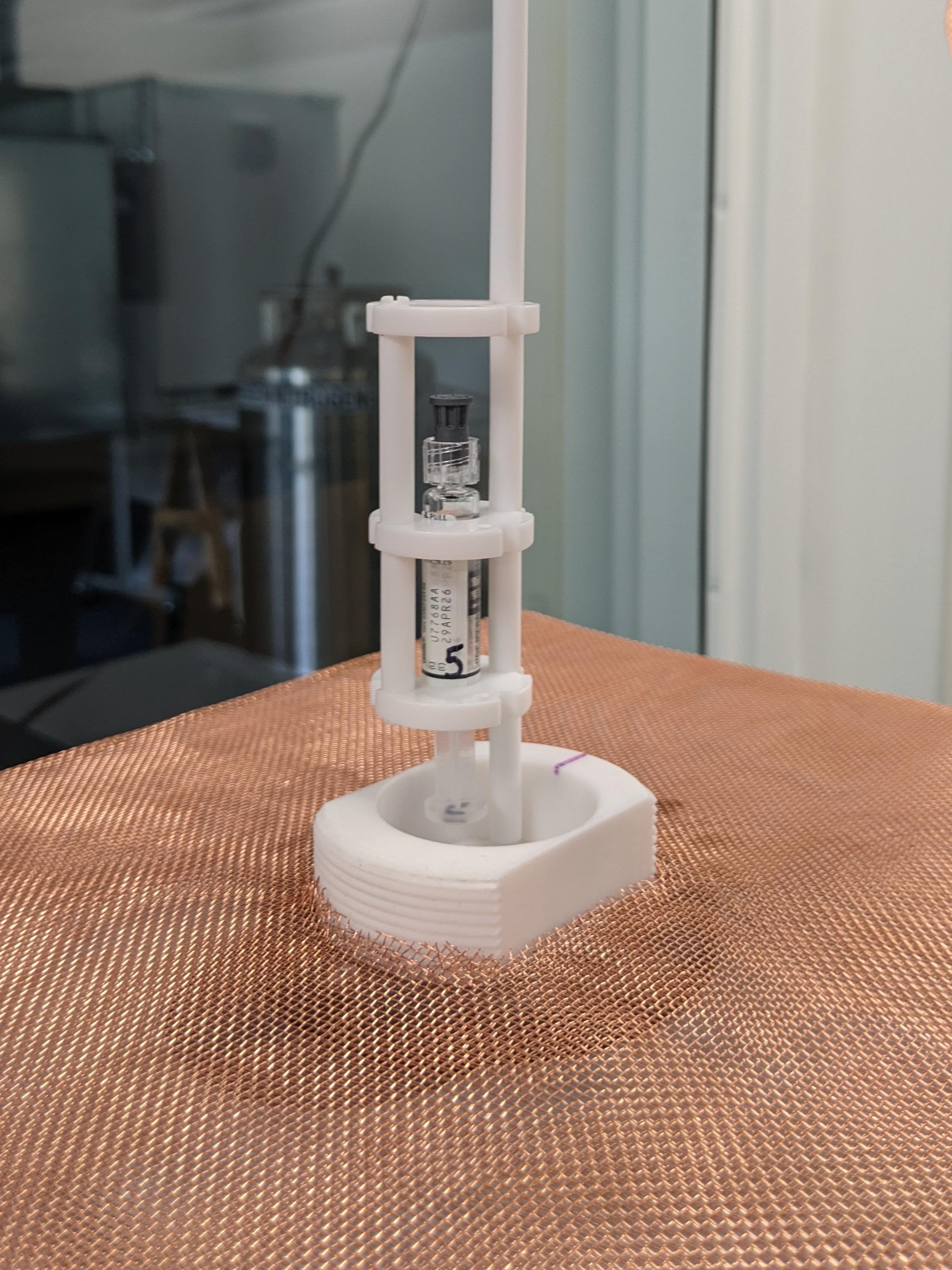 Dr. Bruce Yu, IBBR Fellow and MPower Professor, of the University of Maryland, School of Pharmacy, along with Drs. Marc Taraban, Katharine Briggs, and Pallavi Guha-Biswas have been collaborating with the FDA through the Maryland-Center of Excellence in Regulatory Science and Innovation (M-CERSI) on a regulatory science challenge. The FDA team includes James Coburn, MS; Kirstie Snodderly, MEng; Ashwinkumar Bhirde, PhD, Weiming Ouyang, PhD. The project, initiated early last year, is to evaluate water proton Nuclear Magnetic Resonance (wNMR) technology as a potential product inspection technology for biomanufacturing automation.
Dr. Bruce Yu, IBBR Fellow and MPower Professor, of the University of Maryland, School of Pharmacy, along with Drs. Marc Taraban, Katharine Briggs, and Pallavi Guha-Biswas have been collaborating with the FDA through the Maryland-Center of Excellence in Regulatory Science and Innovation (M-CERSI) on a regulatory science challenge. The FDA team includes James Coburn, MS; Kirstie Snodderly, MEng; Ashwinkumar Bhirde, PhD, Weiming Ouyang, PhD. The project, initiated early last year, is to evaluate water proton Nuclear Magnetic Resonance (wNMR) technology as a potential product inspection technology for biomanufacturing automation.
In biomanufacturing processes, only a small number of products of a batch are opened and tested to ensure quality. This assumes that these few products are representative of the whole batch, and thus overlooks inter-batch variability. Unlike many other measurement technologies, wNMR is non-invasive, so products do not have to be taken out of their vials, syringes, or injection pens. Thus, along with its time-efficient nature, this technology can allow entire batches to be measured, multiple times, and over time.
In this project, three batches of six different products are being tested. The first aim is to compare brand name versus generic products. The second aim is to compare the same product in different presentation forms (vials versus syringes). The third aim is to look at powder vaccines before and after adding diluent. The researchers are currently working on the third batch and are looking to complete the project by August. They hope that their research “could empower manufacturers and regulators to non-invasively assess product quality and biomanufacturing processes using cost-effective wNMR technology.”
This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [Center of Excellence in Regulatory Science & Innovation, U01FD005946] totaling $547,353 from the Office of Counterterrorism and Emerging Threats. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
