Profile

Eric Toth
Associate Research Professor
Toth Group (240) 314-6516 eatoth@umd.eduDr. Eric Toth applies biochemical and biophysical techniques, including X-ray crystallography, to accelerate the development of agents that modulate the function of a wide array of potential therapeutic targets. These efforts include the development of next-generation protein therapeutics, novel vaccines, and small molecule inhibitors of biologically important proteins.
CURRENT RESEARCH
Therapeutic Protein Design and Development
One area of research in Dr. Toth’s laboratory is the engineering of well-characterized proteins to develop new properties for use as therapeutics, sensors, or novel reagents. The development of a protein-based therapeutic directed against cancer-causing Ras protein is a proof-of-principle project currently underway. This project is a collaborative effort conducted by a team composed of Dr. Toth, Dr. John Orban (UMCP Department of Chemistry and Biochemistry) and Dr. Phil Bryan (Potomac Affinity Proteins), with contributions from Dr. Silvia Muro (IBBR) and the Ras Initiative (NCI-Frederick).
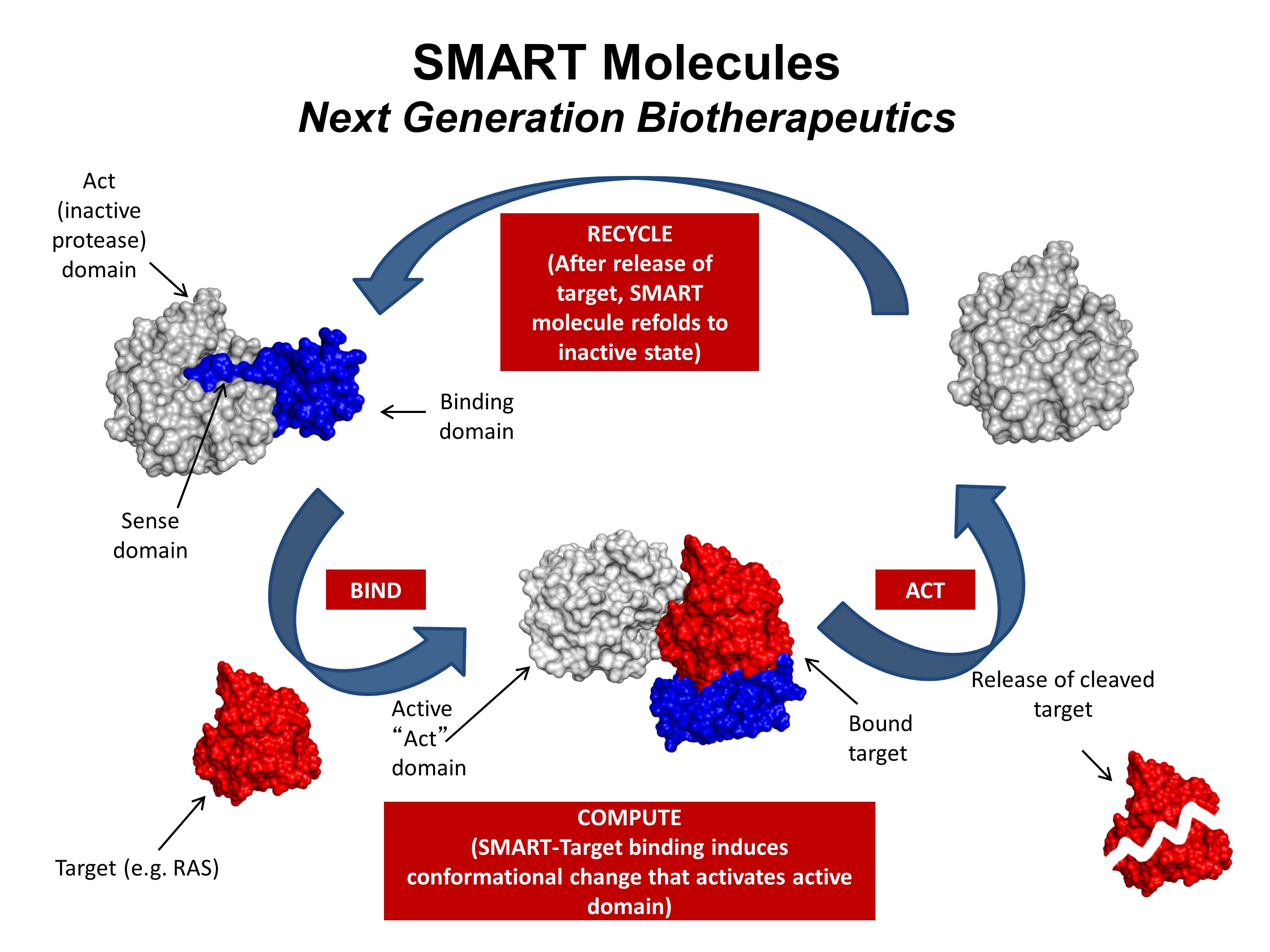
Mutations in one of the three RAS genes are involved in roughly a third of all human cancers, but therapeutic interventions have limited clinical efficacy. The goal of this project is to engineer a highly specific, highly regulated protease capable of destroying mutated human Ras proteins. The long-term objectives are to develop a “smart” therapeutic and to create a platform for future engineering of enzymatic machines to treat drug-resistant cancers and other diseases.
Hepatitis C vaccine
The Toth lab is part of a multi-institutional team, led by IBBR director Dr. Thomas Fuerst, which is developing a vaccine to prevent hepatitis C viral infection. Funded by a $6 million NIH grant, the effort takes a structure-based vaccine design approach to engineer vaccine candidates that elicit a broadly neutralizing immune response. Dr. Toth’s lab is developing novel methods for producing and isolating candidate vaccine antigens for biochemical and immunological testing.
Hepatitis C virus (HCV) is a major cause of severe liver disease and cancer with a global burden of nearly 185 million infected individuals. HCV is an RNA virus that mutates rapidly, making both treatment and vaccine development challenging for this pathogen. While HCV-specific antiviral agents provide effective therapy, a successful treatment does not prevent reinfection. In addition, the high cost of these drugs restricts access, particularly in developing nations where disease burden is greatest, further underscoring the need for a vaccine.
Structure-Based Drug Design
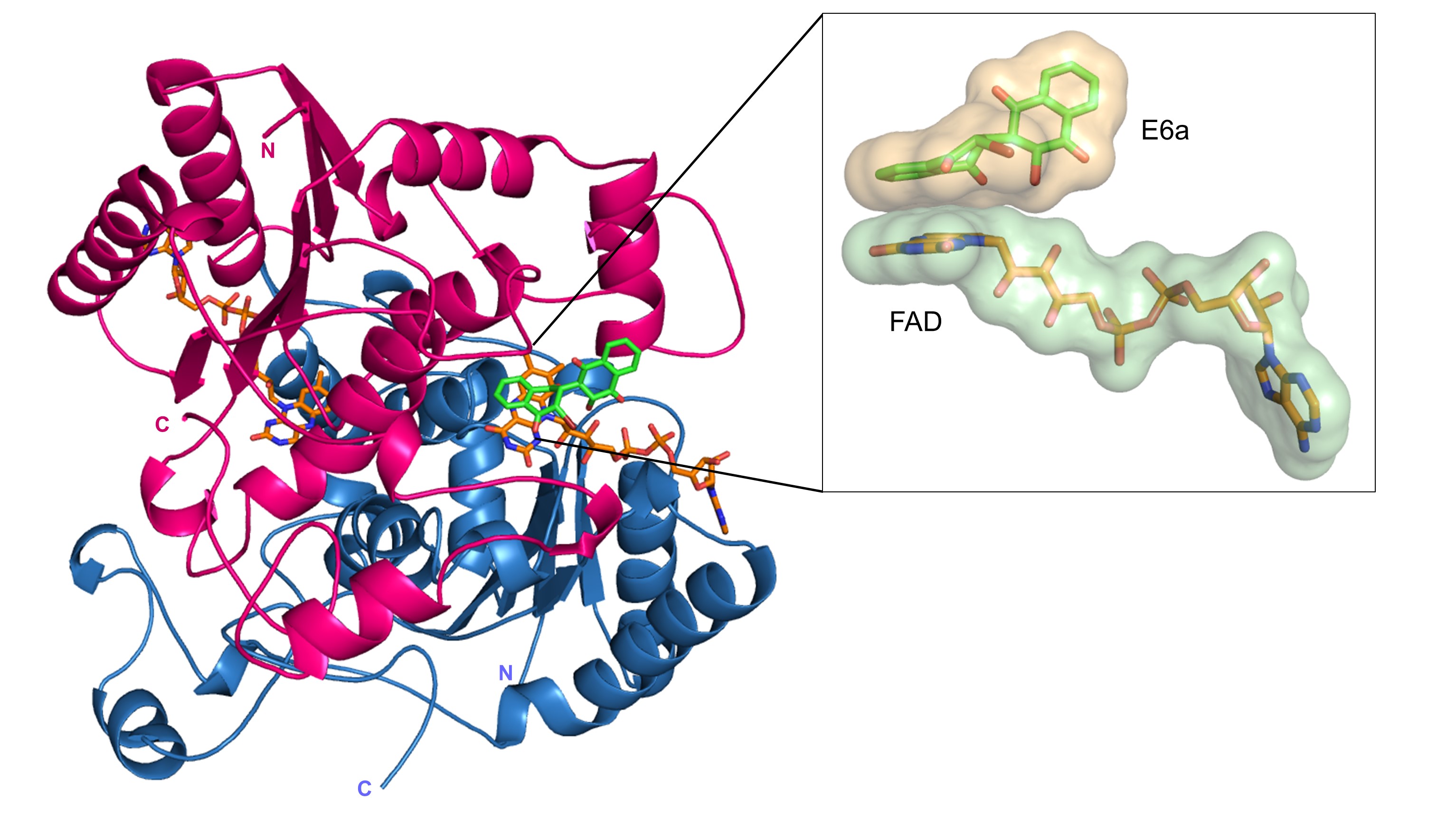
Dr. Toth has led efforts to determine crystal structures of several important drug targets in complex with novel lead compounds aimed at combating cancer and neurological diseases. These efforts include targeting malignant melanoma through disruption of the interaction of p53 with S100B, combating acute myeloid leukemia and other cancers with novel naphthoquinones that inhibit NQO1 (see inset), and targeting neurological disorders by developing inhibitors of the kynurenine pathway of tryptophan degradation.
Publications
- Native-like soluble E1E2 glycoprotein heterodimers on self-assembling protein nanoparticles for hepatitis C virus vaccine design.
- Substrate specificity in a designed RAS-targeting protease is coupled to active site and distal motions.
- mAbClust with AlphaFold 3 avoids hallucinations to define a quaternary broadly neutralizing HCV epitope.
- B cell transcriptomics reveals lasting dysregulation and rapid decline of protective memory after hepatitis C cure.
- Polyphosphazene-Mediated Assembly of TLR4 and TLR7/8 Agonists Enables a Potent Nano-Adjuvant Delivery System for Hepatitis C Virus Vaccine Antigens.
- Enhanced Production of HCV E1E2 Subunit Vaccine Candidates via Protein-Protein Interaction Identification in Glycoengineered CHO Cells.
- Glycoengineering of the hepatitis C virus E2 glycoprotein improves biochemical properties and enhances immunogenicity.
- Enhanced Production of HCV E1E2 Subunit Vaccine Candidates via Protein-Protein Interaction Identification in Glycoengineered CHO cells.
- Native-like soluble E1E2 glycoprotein heterodimers on self-assembling protein nanoparticles for hepatitis C virus vaccine design.
- Cryo-EM structures of HCV E2 glycoprotein bound to neutralizing and non-neutralizing antibodies determined using bivalent Fabs as fiducial markers.
- Glycoengineering of the hepatitis C virus E2 glycoprotein leads to improved biochemical properties and enhanced immunogenicity.
- Virus-Mimicking Polymer Nanocomplexes Co-Assembling HCV E1E2 and Core Proteins with TLR 7/8 Agonist-Synthesis, Characterization, and In Vivo Activity.
- Site-directed neutralizing antibodies targeting structural sites on SARS-CoV-2 spike protein.
- Prospects for developing an Hepatitis C virus E1E2-based nanoparticle vaccine.
- Structure of engineered hepatitis C virus E1E2 ectodomain in complex with neutralizing antibodies.
- Glycosylation shapes the efficacy and safety of diverse protein, gene and cell therapies.
- Skin Vaccination with Ebola Virus Glycoprotein Using a Polyphosphazene-Based Microneedle Patch Protects Mice against Lethal Challenge.
- Structure-based design of N-substituted 1-hydroxy-4-sulfamoyl-2-naphthoates as selective inhibitors of the Mcl-1 oncoprotein.
- A direct interaction between NQO1 and a chemotherapeutic dimeric naphthoquinone.
- Hydroxylated Dimeric Naphthoquinones Increase the Generation of Reactive Oxygen Species, Induce Apoptosis of Acute Myeloid Leukemia Cells and Are Not Substrates of the Multidrug Resistance Proteins ABCB1 and ABCG2.
- Small Molecule Inhibitors of Ca(2+)-S100B Reveal Two Protein Conformations.
- Structure of human apurinic/apyrimidinic endonuclease 1 with the essential Mg2+ cofactor.
- Coordination of MYH DNA glycosylase and APE1 endonuclease activities via physical interactions.
- The crystal structure of human quinolinic acid phosphoribosyltransferase in complex with its inhibitor phthalic acid.
- A unique IBMPFD-related P97/VCP mutation with differential binding pattern and subcellular localization.
- Structure-Based Discovery of a Novel Pentamidine-Related Inhibitor of the Calcium-Binding Protein S100B.
- Hsp70 is a novel posttranscriptional regulator of gene expression that binds and stabilizes selected mRNAs containing AU-rich elements.
- Target binding to S100B reduces dynamic properties and increases Ca(2+)-binding affinity for wild type and EF-hand mutant proteins.
- Crystal structure of human methyl-binding domain IV glycosylase bound to abasic DNA.
- Yeast nuclear RNA processing.
- In vitro screening and structural characterization of inhibitors of the S100B-p53 interaction.
- Unique properties of the Mtr4p-poly(A) complex suggest a role in substrate targeting.
- Alternatively expressed domains of AU-rich element RNA-binding protein 1 (AUF1) regulate RNA-binding affinity, RNA-induced protein oligomerization, and the local conformation of bound RNA ligands.
- A structural hinge in eukaryotic MutY homologues mediates catalytic activity and Rad9-Rad1-Hus1 checkpoint complex interactions.
- Contributions of the histidine side chain and the N-terminal alpha-amino group to the binding thermodynamics of oligopeptides to nucleic acids as a function of pH.
- The effects of CapZ peptide (TRTK-12) binding to S100B-Ca2+ as examined by NMR and X-ray crystallography.
