News Stories

IBBR Graduate Student Contributes to Breakthrough Study on Chestnut Tree Restoration Published in Science
A new study published February 12, 2026, in Science, one of the world’s leading journals in scientific research and discovery, demonstrates that modern genomic tools can accelerate restoration of the American chestnut tree. The study, entitled “Genomic Approaches to Accelerate American Chestnut Restoration,” describes the genetics of disease resistance in...
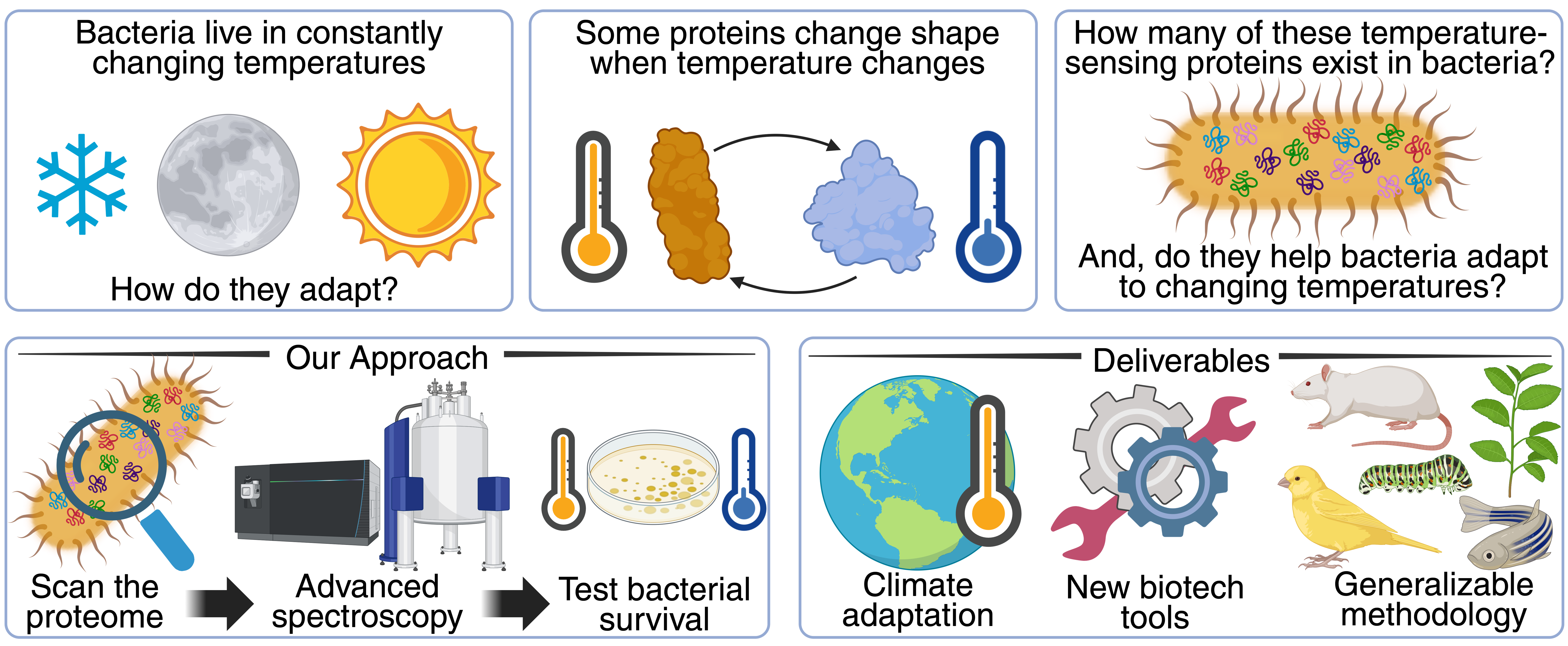
$1.2M Keck Foundation Award Supports IBBR Research on Shape-Shifting Proteins
IBBR is proud to congratulate Dr. John Orban’s lab, along with collaborators from UC-Merced and Caltech, on receiving a $1.2 million research award from the W.M. Keck Foundation. This prestigious grant will propel an ongoing project exploring metamorphic proteins; proteins that can adopt more than one folded state. Unlike endotherms...
.png)
Manghrani Wins Poster Award at the Annual Oligonucleotide Therapeutics Society Meeting
Akanksha Manghrani, a NIST–IBBR postdoctoral research associate in the Biomolecular Measurement Division of the Materials and Measurements Laboratory, received a poster award at the Annual Oligonucleotide Therapeutics Society (OTS) Meeting held in Budapest, Hungary this October. The OTS annual meeting is the premier global conference for researchers and industry leaders...
About IBBR
IBBR is a joint research enterprise of the University of Maryland, College Park, the University of Maryland, Baltimore, and the National Institute of Standards and Technology.
IBBR leverages state-of-art integrative methods for bioanalytical, biophysical and structural characterization of biomolecules: cryo-electron microscopy, nuclear magnetic resonance, x-ray crystallography, small angle neutron and x-ray scattering and mass spectrometry.
IBBR researchers seek to advance therapeutic development, biomanufacturing, and state-of-the-art measurement technologies, to support accelerated delivery of safe and effective medicines to the public.
IBBR is a major initiative and supported in part by the University of Maryland Strategic Partnership: MPowering the State (MPower) , an initiative designed to achieve innovation and impact through collaboration.
Connecting
IBBR Commons
Sophisticated state-of-the-art instrumentation and facilities, and in-house expertise located in shared space and dedicated to advance research, support collaboration and foster innovation of methods. Instrumentation and facilities include tools for high-resolution structural biology, bioanalytical and biophysical measurement, protein engineering and cell culture, advanced computation including artificial intelligence and deep learning methods, and general laboratory services. These capabilities and advanced training are available to IBBR scientists and collaborators.
IBBR Postdoc Program
The IBBR Postdoc Program (IPP) focuses on collaborative research involving basic science and technology development that advances therapeutic development, vaccine development, and biomanufacturing. IPP Fellow project teams are designed with a combination of the IPP Fellow career goals and priorities of project mentors who can be from academic, government, and/or industrial laboratories throughout the University of Maryland, NIST and the I-270 corridor.
NMRPipe
IBBR is home to NMRPipe, a popular collection of programs and scripts for manipulating multidimensional Nuclear Magnetic Resonance (NMR) data. The use of NMRPipe is noted in roughly 40% of all NMR structures accepted into the Protein Data Bank.

Upcoming Events
Seminar: "Antiviral immunity by nucleotide second messengers in bacteria"
Presentation given by
Uday Tak
University of Virginia
BMD Staff Seminar
NIST Group Meeting; Frank Delaglio
Recent Publications
TEpiNom: A computational framework integrating population data to prioritize plasmodium falciparum T cell epitopes.
Developing a highly effective malaria vaccine remains challenging due to Plasmodium falciparum's antigenic diversity and human leukocyte antigen (HLA) polymorphisms, which complicate antigen...
Using machine learning to predict and analyze complex trait diseases: Lessons from a simple abstract model.
The ability to predict individual genetic susceptibility to a complex trait disease is a major challenge in modern medicine. One approach to addressing this challenge utilizes an additive...
Transgene-free genome editing in citrus and poplar meristem tissues via biolistic ribonucleoprotein delivery of CRISPR-Cas9.
Biolistic particle bombardment was used to deliver CRISPR-Cas9 ribonucleoprotein complexes (RNP) into the shoot apical meristem tissue of citrus and axillary meristem tissue of poplar, generating...
Mediated Electrochemical Probing and Machine Learning for Cysteine and Reduced Monoclonal Antibody Quantification.
Biopharmaceutical manufacturing requires robust analytics and process controls throughout production to insure high yield of quality products. New methodologies for rapidly accessing and...
Native-like soluble E1E2 glycoprotein heterodimers on self-assembling protein nanoparticles for hepatitis C virus vaccine design.
Hepatitis C virus (HCV) is a leading cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma worldwide. Development of an E1E2-based HCV vaccine has been hindered by the difficulty...