Profile

Frank Delaglio
Dr. Frank Delaglio develops computational methods for extracting useful information from Nuclear Magnetic Resonance (NMR) data to support development and manufacturing of protein drugs, live cell therapies, and vaccines, and to support basic research in structural biology. His current focus is on development of approaches for characterization of the high order structure of biologics, so that these important therapeutics can continue to be safe and effective, and become more available and affordable. In the NMR community, Dr. Delaglio is best known as the developer of the NMRPipe software system (http://www.ibbr.umd.edu/nmrpipe), which has been cited over 15,000 times in peer-reviewed literature and has been used to help generate roughly 40% of the protein structures ever measured by NMR.
 Dr. Delaglio’s areas of interest include multidimensional spectral processing and analysis, chemometrics and machine learning, data visualization, and NMR homology approaches to biomolecular structure determination. His work always includes the goal of producing practical, working software. He has made more than 200 lectures and tutorials in 15 countries, including 20+ years as advanced course instructor for the European Molecular Biology Organization (EMBO) and the UCONN Center for Biomolecular NMR Data Processing and Analysis (nmrbox.org). He shared a 2022 Department of Commerce Silver Medal for contributions to biomanufacuring, and was awarded the 2024 Americal Chemical Society Award in Chemical Instrumentation.
Dr. Delaglio’s areas of interest include multidimensional spectral processing and analysis, chemometrics and machine learning, data visualization, and NMR homology approaches to biomolecular structure determination. His work always includes the goal of producing practical, working software. He has made more than 200 lectures and tutorials in 15 countries, including 20+ years as advanced course instructor for the European Molecular Biology Organization (EMBO) and the UCONN Center for Biomolecular NMR Data Processing and Analysis (nmrbox.org). He shared a 2022 Department of Commerce Silver Medal for contributions to biomanufacuring, and was awarded the 2024 Americal Chemical Society Award in Chemical Instrumentation.
CURRENT RESEARCH
Characterization of Biologics by NMR, Chemometrics, and Machine Learning
With annual sales exceeding $150 billion, growth of biologic therapeutics is outpacing that of small-molecule drugs, and the development, manufacture, and delivery of biologics present very different challenges. Among biologics, monoclonal antibodies (mAbs) have become particularly attractive for drug development, since it is possible to select and duplicate antibodies that bind with high affinity and specificity to most any target, and since biomanufacturing platforms for mAbs can be reused for more than one therapeutic.
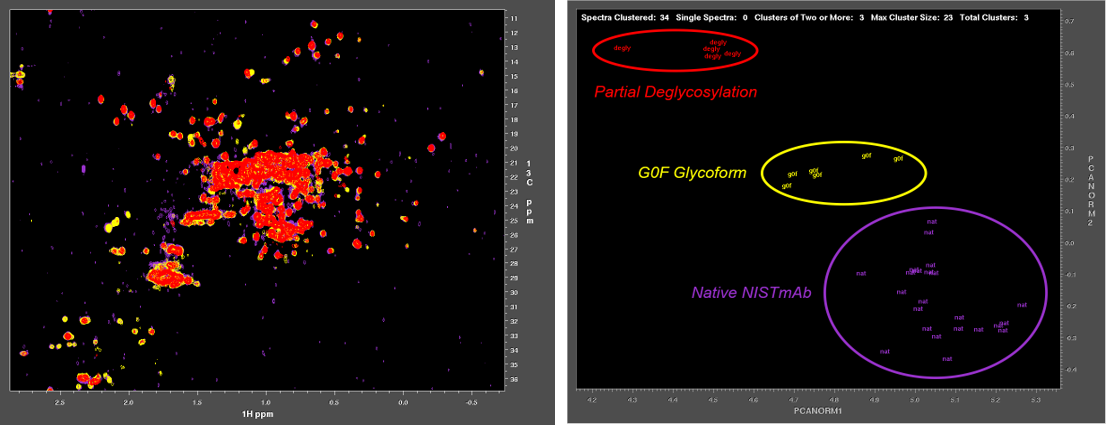
Characterization of biologics and their formulations requires monitoring high order structure (HOS), since misfolding or aggregation can lead to loss of efficacy or harmful immune responses. Nuclear Magnetic Resonance (NMR) is a powerful multi-attribute method that can be applied directly to intact biologics and their formulations to meet this important measurement need. However, typical biomolecular NMR applications use isotopically enriched samples, require long measurement times, and need extensive and often subjective interactive analysis by an expert in order to extract structural information. Previous work has shown that fast NMR measurement techniques combined with Non-Uniform Sampling (NUS) can generate practical two-dimensional 1H/13C spectra at natural isotopic abundance for molecules as large as intact monoclonal antibodies. Leveraging the IgG1k NIST reference mAb (NISTmAb), Dr. Delaglio’s current research aims to reveal and classify small variations of structure and interaction by direct computational analysis of the shapes of NMR spectra as an alternative to interactive analysis and assignment of spectral features. This paves the way for NMR characterization of biologics via chemometrics and machine learning that is both objective and automated. The Delaglio Group is in the early stages of applying low field NMR to characterize bioreactions for the production of mAbs and live cell therapeutics, and accordingly the group is starting to develop computational methods for metabolomics, and for solid state NMR. Frank also provides computational support and methods for water relaxometry of injectable drug products, including an in-progress multilab study with 6 vendor participants.
Machine Learning Approaches to Spectral Analysis
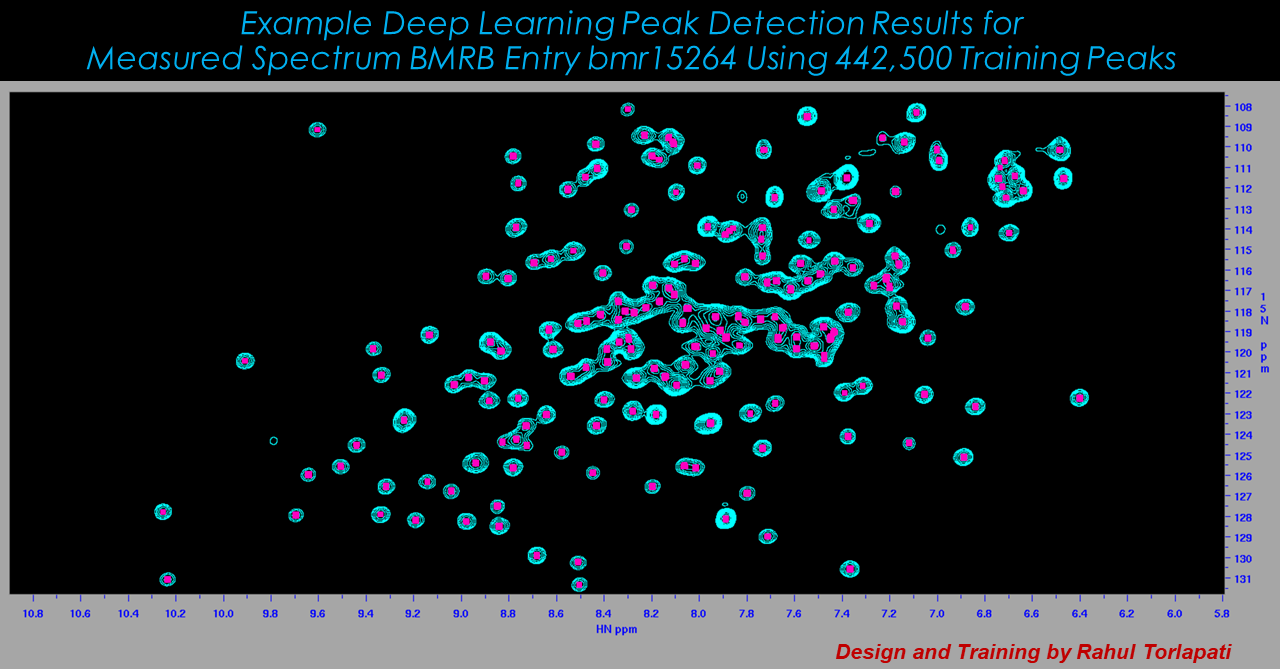
While workflows vary, a common aspect of most any NMR analysis is to identify spectral features ("peaks"). While automated software can do most of this work, a typical analysis requires that peak detection is curated by an expert. This is subjective and can be extremely time-consuming, since individual spectra can have hundreds or thousands of peaks, and applications can involve dozens or hundreds of spectra. Other requirements common to many NMR workflows include identifying peaks that are related to each other, following changes in peaks over a collection of related spectra such as a time series, or gauging the similarity between two spectra. In all of these cases, a human analyst makes high-quality decisions by inspecting spectral graphics in the form of 1D plots and 2D contour plots. The Delaglio Group explores application of computer vision methods to these analysis tasks.
Publications
- Reducing the measurement time of exact NOEs by non-uniform sampling.
- Principal component analysis for automated classification of 2D spectra and interferograms of protein therapeutics: influence of noise, reconstruction details, and data preparation.
- Assessment of the Higher-Order Structure of Formulated Monoclonal Antibody Therapeutics by 2D Methyl Correlated NMR and Principal Component Analysis.
- Comparative Analysis of One-Dimensional Protein Fingerprint by Line Shape Enhancement and Two-Dimensional 1H,13C Methyl NMR Methods for Characterization of the Higher Order Structure of IgG1 Monoclonal Antibodies.
- Best Practices in Utilization of 2D-NMR Spectral Data as the Input for Chemometric Analysis in Biopharmaceutical Applications.
- Chemometric Outlier Classification of 2D-NMR Spectra to Enable Higher Order Structure Characterization of Protein Therapeutics.
- Enabling adoption of 2D-NMR for the higher order structure assessment of monoclonal antibody therapeutics.
- Selective suppression of excipient signals in 2D 1H-13C methyl spectra of biopharmaceutical products.
- Non-Uniform Sampling for All: More NMR Spectral Quality, Less Measurement Time.
- Nonuniform sampling in multidimensional NMR for improving spectral sensitivity.
- Solution structure of (gamma)S-crystallin by molecular fragment replacement NMR.
- Molecular fragment replacement approach to protein structure determination by chemical shift and dipolar homology database mining.
- The utility of residual dipolar couplings in detecting motion in carbohydrates: application to sucrose.
