Profile

John Marino
Dr. John Marino’s research is focused on developing nuclear magnetic resonance (NMR) and other biophysical measurements to accurately and precisely define the conformational structure, stability, and dynamics of biomolecules and their interactions at a molecular level.
In addition to enabling fundamental insights into biomolecular structure and function, Dr. Marino’s work provides innovative, yet practical methods that can form the basis for a robust measurement infrastructure that supports biopharmaceutical development and regulation.
CURRENT RESEARCH
Biopharmaceuticals represent over 40% of the medicines in development today, making this industry a valuable and important sector of the US economy. A key focus is on the development of advanced techniques for accurate and precise characterization of higher-order-structure (HOS) in biotherapeutics, particularly monoclonal antibodies, which are sought by industry and regulators to establish consistency in drug manufacturing, detect process-related drug-product variations and compare biosimilars to innovator reference products.
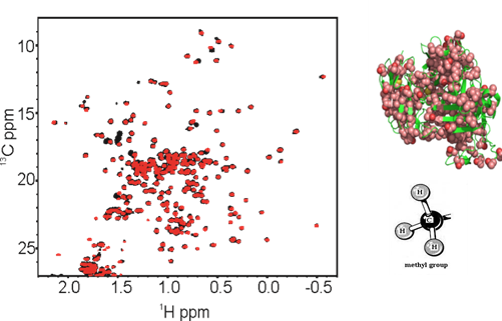
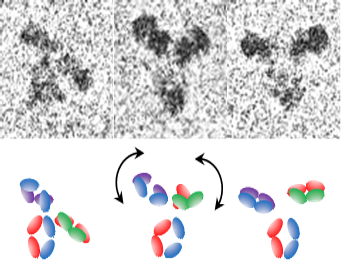
2D NMR for Biopharmaceutical Structure Assessment
Correct folding of biopharmaceuticals is critical for drug efficacy, with misfolding also impacting drug safety by eliciting unwanted immune and/or other off-target responses. High-resolution measurements of the structure(s) of protein therapeutics provide important tools for establishing consistency in drug manufacturing and detection of drug product changes resulting from modifications in the manufacturing process. NMR, a high-resolution and information-rich technique, is being applied as a tool for structural assessment of monoclonal antibodies (mAbs) biopharmaceuticals. This method is highly sensitive to molecular structure alterations that result from mutations, misfoldings, or contaminants. Experimental approaches are being developed to obtain structural 'fingerprints' of the bioactive form of protein therapeutics, as well as detection of variants, at atomic resolution. In parallel, computational alternatives (e.g., chemometrics and machine learning) to interactive analysis of spectral features are being explored.
Measurements of Biopharmaceuticals using Cryogenic Electron Microscopy (cryo-EM)
In biopharmaceutical applications, analysis of small, asymmetric molecules, which exhibit inherent flexibility, is required. Cryo-EM approaches are being explored that will enable high-resolution (better than 0.3 nm) structural characterization of flexible, protein-based drugs that are below a molecular weight limit of ~200 kDa. To address current limitations of low contrast and the challenges with alignment and averaging of small, flexible biomolecules, new strategies for bioengineering sample platforms, image analysis, and mathematical modeling are being pursued.
Publications
- Contributions from ClpS surface residues in modulating N-terminal peptide binding and their implications for NAAB development.
- Backbone NMR assignment of the yeast expressed Fab fragment of the NISTmAb reference antibody.
- Correlated analytical and functional evaluation of higher order structure perturbations from oxidation of NISTmAb.
- Structural Fingerprinting of Antisense Oligonucleotide Therapeutics by Solution NMR Spectroscopy.
- Selective C-Terminal Conjugation of Protease-Derived Native Peptides for Proteomic Measurements.
- Structural Fingerprinting of Short Interfering RNA Therapeutics by Solution Nuclear Magnetic Resonance Spectroscopy.
- Excipient Innovation Through Precompetitive Research.
- Highly conserved s2m element of SARS-CoV-2 dimerizes via a kissing complex and interacts with host miRNA-1307-3p.
- The emerging landscape of single-molecule protein sequencing technologies.
- Grand Challenges in Pharmaceutical Research Series: Ridding the Cold Chain for Biologics.
- Principal component analysis for automated classification of 2D spectra and interferograms of protein therapeutics: influence of noise, reconstruction details, and data preparation.
- Leveraging nature's biomolecular designs in next-generation protein sequencing reagent development.
- Assessment of the Higher-Order Structure of Formulated Monoclonal Antibody Therapeutics by 2D Methyl Correlated NMR and Principal Component Analysis.
- Comparative Analysis of One-Dimensional Protein Fingerprint by Line Shape Enhancement and Two-Dimensional 1H,13C Methyl NMR Methods for Characterization of the Higher Order Structure of IgG1 Monoclonal Antibodies.
- Best Practices in Utilization of 2D-NMR Spectral Data as the Input for Chemometric Analysis in Biopharmaceutical Applications.
- Chemometric Outlier Classification of 2D-NMR Spectra to Enable Higher Order Structure Characterization of Protein Therapeutics.
- Structure and Dynamics of a Site-Specific Labeled Fc Fragment with Altered Effector Functions.
- 2D J-correlated proton NMR experiments for structural fingerprinting of biotherapeutics.
- Single-Molecule 3D Images of "Hole-Hole" IgG1 Homodimers by Individual-Particle Electron Tomography.
- Engineering ClpS for selective and enhanced N-terminal amino acid binding.
- A small protein inhibits proliferating cell nuclear antigen by breaking the DNA clamp.
- A Molecular Model for Lithium's Bioactive Form.
- A small protein inhibits proliferating cell nuclear antigen by breaking the DNA clamp.
- Precision and robustness of 2D-NMR for structure assessment of filgrastim biosimilars.
- Applying Thymine Isostere 2,4-Difluoro-5-Methylbenzene as a NMR Assignment Tool and Probe of Homopyrimidine/Homopurine Tract Structural Dynamics.
- Application of Natural Isotopic Abundance ¹H-¹³C- and ¹H-¹⁵N-Correlated Two-Dimensional NMR for Evaluation of the Structure of Protein Therapeutics.
- Revisiting plus-strand DNA synthesis in retroviruses and long terminal repeat retrotransposons: dynamics of enzyme: substrate interactions.
- SHAMS: combining chemical modification of RNA with mass spectrometry to examine polypurine tract-containing RNA/DNA hybrids.
- Dissecting structural transitions in the HIV-1 dimerization initiation site RNA using 2-aminopurine fluorescence.
- Probing anomalous structural features in polypurine tract-containing RNA-DNA hybrids with neomycin B.
- Synthesis of HIV-1 Psi-site RNA sequences with site specific incorporation of the fluorescent base analog 2-aminopurine.
- Structural probing of the HIV-1 polypurine tract RNA:DNA hybrid using classic nucleic acid ligands.
- New crystal structures of ColE1 Rom and variants resulting from mutation of a surface exposed residue: Implications for RNA-recognition.
- Examining Ty3 polypurine tract structure and function by nucleoside analog interference.
- A ribose sugar conformational switch in the LTR-retrotransposon Ty3 polypurine tract-containing RNA/DNA hybrid.
- Heterotrimeric G-protein alpha-subunit adopts a "preactivated" conformation when associated with betagamma-subunits.
- Bacterial expression and one-step purification of an isotope-labeled heterotrimeric G-protein alpha-subunit.
- JE-TROSY: combined J- and TROSY-spectroscopy for the measurement of one-bond couplings in macromolecules.
- Proflavine acts as a Rev inhibitor by targeting the high-affinity Rev binding site of the Rev responsive element of HIV-1.
- Evidence for structural changes in carboxyl-terminal peptides of transducin alpha-subunit upon binding a soluble mimic of light-activated rhodopsin.
