Profile

Yuxing Li
Professor
Yuxing Li Group (240) 314-6332 yuxingli@umd.eduDr. Yuxing Li’s lab studies how B cells, a critical part of the immune system, respond to viral infection, and applies these findings toward antibody discovery and the development of vaccines and therapeutics to treat viral infections.
Dr. Li’s work has focused on defining broadly neutralizing antibody responses elicited by HIV-1 envelope glycoproteins during natural infections and in animal models. These findings contributed substantially to the in-depth understanding of HIV broadly neutralizing antibody response and the subsequent discovery of broadly neutralizing monoclonal antibodies targeting the HIV envelope glycoprotein receptor binding site, and have important implications for vaccine and immunotherapeutics development.
CURRENT RESEARCH
Development of B cell response targeting HIV-1 envelope glycoproteins (Env)
Dr Li’s work is focused on finding novel immunization regimens to better elicit broadly neutralizing antibodies. Dr. Li’s laboratory aims to better understand the mechanism underlying protective immunity and contribute to the development of a broadly effective HIV-1 vaccine.
Antigen-specific multi-color single B cell sorting and monoclonal antibody cloning
The Li lab is using rational design of HIV-1 (Env) immunogens and immunization regimens to analyze B cell and antibody responses with cutting-edge techniques. The Li lab is working to better define the B cell response to experimental HIV-1 vaccines toward discovering novel antibodies and gain insight into viral infection and vaccination.
Development of novel anti-viral immunotherapeutics by structure-based antibody engineering
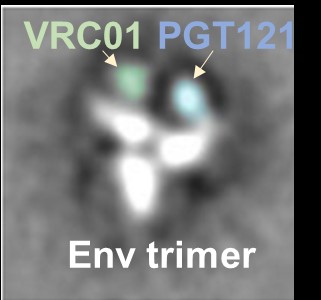
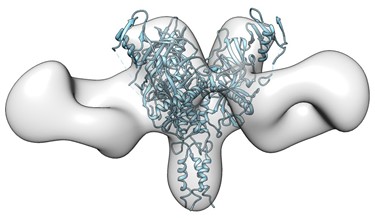
Recently, Dr. Li’s laboratory successfully created a bi-specific antibody that combined functional epitope-binding moieties from two broadly neutralizing antibodies, VRC01 and PGT 121, which simultaneously binds two epitopes within one Env trimer and neutralizes >96% of circulating
HIV-1 isolates. Multi-specific antibodies developed based on this design demonstrated improved antiviral breadth and potency.
In close collaboration with Integrated Biotherapeutics, Inc., the Li lab successfully isolated a broadly neutralizing antibody, CA45, from an Ebola virus vaccine candidate, immunized, non-human primate animal and developed a pan-Ebolavirus therapeutic antibody cocktail for a pre-clinical trial in a non-human primate model. Therapeutic antibodies for Ebola virus infection were only protective against the Zaire Ebola strain but not other related filoviruses. The lab’s research focus is on understanding B cell responses to the filovirus glycoproteins to inform vaccine design to develop a broadly protective antibody response.
Publications
- One dose of COVID-19 nanoparticle vaccine REVC-128 protects against SARS-CoV-2 challenge at two weeks post-immunization.
- Pre-vaccination and early B cell signatures predict antibody response to SARS-CoV-2 mRNA vaccine.
- Prominent Neutralizing Antibody Response Targeting the Ebolavirus Glycoprotein Subunit Interface Elicited by Immunization.
- Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation.
- Antigen-Specific Single B Cell Sorting and Monoclonal Antibody Cloning in Guinea Pigs.
- The HIV-1 Envelope Glycoprotein C3/V4 Region Defines a Prevalent Neutralization Epitope following Immunization.
- Post-exposure immunotherapy for two ebolaviruses and Marburg virus in nonhuman primates.
- An HIV-1 Env-Antibody Complex Focuses Antibody Responses to Conserved Neutralizing Epitopes.
- Maturational characteristics of HIV-specific antibodies in viremic individuals.
- Maintenance of HIV-Specific Memory B-Cell Responses in Elite Controllers Despite Low Viral Burdens.
- Key gp120 Glycans Pose Roadblocks to the Rapid Development of VRC01-Class Antibodies in an HIV-1-Infected Chinese Donor.
- High-Resolution Longitudinal Study of HIV-1 Env Vaccine-Elicited B Cell Responses to the Virus Primary Receptor Binding Site Reveals Affinity Maturation and Clonal Persistence.
- HIV-1 Vaccine-elicited Antibodies Reverted to Their Inferred Naive Germline Reveal Associations between Binding Affinity and in vivo Activation.
- Reversible Reprogramming of Circulating Memory T Follicular Helper Cell Function during Chronic HIV Infection.
- Rhesus Macaque B-Cell Responses to an HIV-1 Trimer Vaccine Revealed by Unbiased Longitudinal Repertoire Analysis.
- Diverse antibody genetic and recognition properties revealed following HIV-1 envelope glycoprotein immunization.
- Bone marrow plasma cells are a primary source of serum HIV-1-specific antibodies in chronically infected individuals.
- HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies.
- HIV-1 receptor binding site-directed antibodies using a VH1-2 gene segment orthologue are activated by Env trimer immunization.
- Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals.
- Glycosylation, hypogammaglobulinemia, and resistance to viral infections.
- Single-cell and deep sequencing of IgG-switched macaque B cells reveal a diverse Ig repertoire following immunization.
- Vaccine-elicited primate antibodies use a distinct approach to the HIV-1 primary receptor binding site informing vaccine redesign.
- Proof of principle for epitope-focused vaccine design.
- Identification and characterization of a broadly cross-reactive HIV-1 human monoclonal antibody that binds to both gp120 and gp41.
- HIV-1 neutralizing antibodies display dual recognition of the primary and coreceptor binding sites and preferential binding to fully cleaved envelope glycoproteins.
- High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site.
- Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site.
- Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates.
- Selective expansion of HIV-1 envelope glycoprotein-specific B cell subsets recognizing distinct structural elements following immunization.
- Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site.
- Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals.
- Broad HIV-1 neutralization mediated by CD4-binding site antibodies.
- Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity.
- Immune selection of equine infectious anemia virus env variants during the long-term inapparent stage of disease.
