IBBR Study Advances Hepatitis C Vaccine Development
Mon, Oct 9, 2023
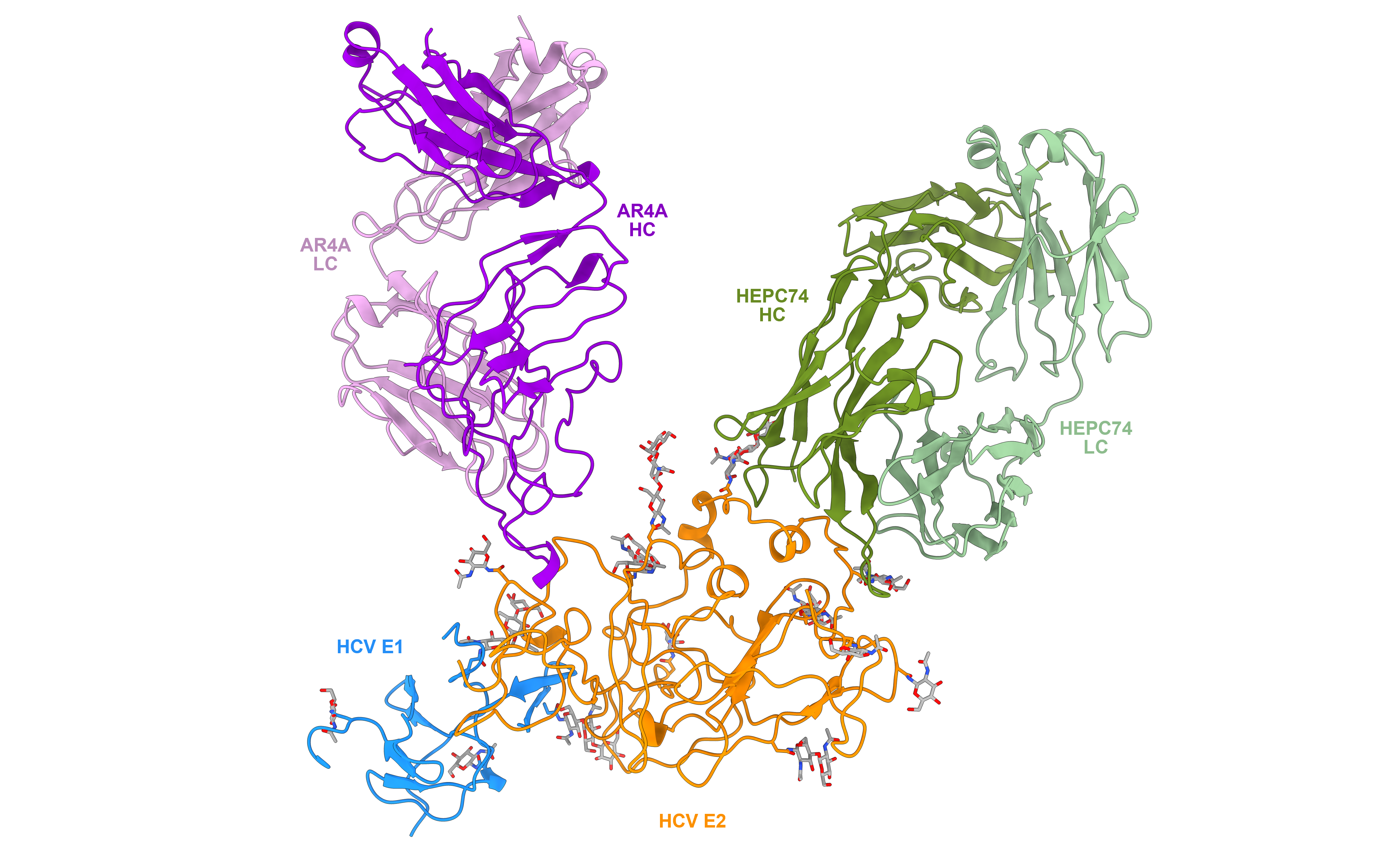
Hepatitis C virus (HCV) poses a tremendous global health concern as a cause of hepatocellular carcinoma and other liver diseases, with over 58 million people infected globally and 1.5 million new infections each year. Current direct-acting antiviral drugs are effective cures but expensive and subject to viral resistance variants, posing accessibility issues and making it difficult to control infection worldwide. Moreover, many people don’t know they are infected, and patients can be reinfected. An HCV vaccine targeting the E1E2 surface glycoprotein, the main target of HCV-neutralizing antibodies, is therefore considered the most effective approach for limiting infection rates. However, various challenges have complicated efforts in developing a vaccine, including high sequence diversity (over 6 genotypes and 90 subtypes) and difficulties in producing soluble forms of the E1E2 glycoprotein that retain native features necessary for induction of effective immune responses.
In the face of this challenge, investigators at IBBR, including Drs. Gilad Ofek, Thomas Fuerst, Eric Toth, and Brian Pierce previously developed a secreted, soluble form of the glycoprotein, termed sE1E2.SZ, that retained recognition by HCV antibodies through addition of a stabilizing scaffold. In the present study, Dr. Gilad Ofek (Assistant Professor, Department of Cell Biology and Molecular Genetics, University of Maryland College Park and IBBR Fellow), Dr. Matthew Metcalf (a post-doctoral fellow in his laboratory) and colleagues, report the cryoEM structure of sE1E2.SZ in complex with broadly neutralizing antibodies. This novel structure from the Ofek group is extremely impactful and reveals that sE1E2.SZ maintains native structural features of the glycoprotein critical for its use as an effective vaccine antigen. The study also identifies highly conserved regions on E1E2 that can broaden vaccine efficacy. The determined structure advances the understanding of E1E2 and enables further rational design of secreted forms of E1E2 for vaccine development. This work was recently published in Nature Communications.
Development of an HCV vaccine is crucial and an important mandate of the World Health Organization. Dr. Ofek together, in this study, in collaboration with Dr. Fuerst at IBBR are generating great new advancements towards understanding HCV and have received significant funding from the National Institutes of Health to support these advances.
