NIST-IBBR Researchers Awarded Department of Commerce Gold Medal for developing the NISTmAb Standard
Wed, Oct 11, 2017

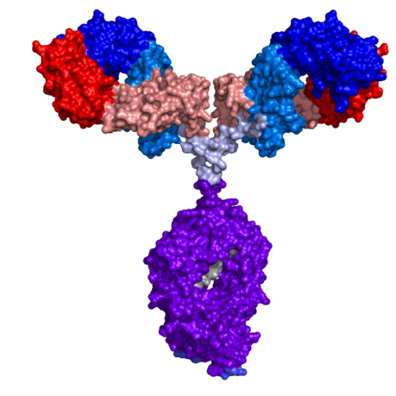
October 11, 2017 -- NIST-IBBR scientists share in 2017 Department of Commerce (DOC) Gold Medal Award, the highest honor award bestowed by the United States Department of Commerce. John Schiel, Abigail Turner, and Catherine Mouchahoir were recognized with their NIST colleagues Srivalli Telikepalli and Paul Derose for developing NISTmAb, industry’s first monoclonal antibody reference material and benchmarking tool for the manufacturing of life-saving protein drugs. This prestigious award was presented to the recipients at the 69 Annual DOC Awards Ceremony on September 26, 2017, in Washington, DC, by the United States Secretary of Commerce, Wilbur Ross.
The DOC Gold Medal Award criteria require that “the impact of the accomplishment must be truly exceptional and reflect the highest level of achievement in the Department.” The importance and impact of the NISTmAb, an exhaustively analyzed antibody protein – consisting of more than 20,000 atoms – certainly meets those lofty goals. This achievement has provided a standard benchmark for ensuring the quality of mAb (monoclonal antibody) therapeutics, a class of drug that is far more complex than small molecule drugs and is becoming increasingly important in the treatment of serious and life-threatening medical conditions.
Small molecule drugs rely on chemically-manufactured active substances, thus making reproduction, analysis, and manufacturing of the molecule more predictable and reliable. Since mAbs are produced by cells, reproduced copies may not be exact duplicates. This differentiation means that all manufactured batches of mAb therapeutics must be rigorously tested and analyzed to be sure that they are retaining their efficacy, purity, and safety. “The NISTmAb should help in answering a simple, yet critical, question that can consume a disproportionate amount of time when deviations arise with testing; is it the sample or the method that is varying?” said Michael Tarlov, Chief, Biomolecular Measurement Division, NIST.
The utility of the NISTmAb as an analytical benchmarking tool was first demonstrated in a collaborative study involving over 100 participants from industry, regulatory, and international university research teams that used analytical methods to thoroughly measure and characterize the mAb. Their findings were documented in a three-volume book series published by the American Chemical Society (ACS). This compiled data, documentation and reference material will be an invaluable resource for ensuring unambiguous analytical testing and should enable drug manufacturers to meet FDA regulations and hasten the availability of new, beneficial pharmaceuticals to the marketplace.
-----
Inquiries: communications@ibbr.umd.edu
