Novel shape-shifting proteins may provide deeper insights into the folding code
Wed, Feb 8, 2023
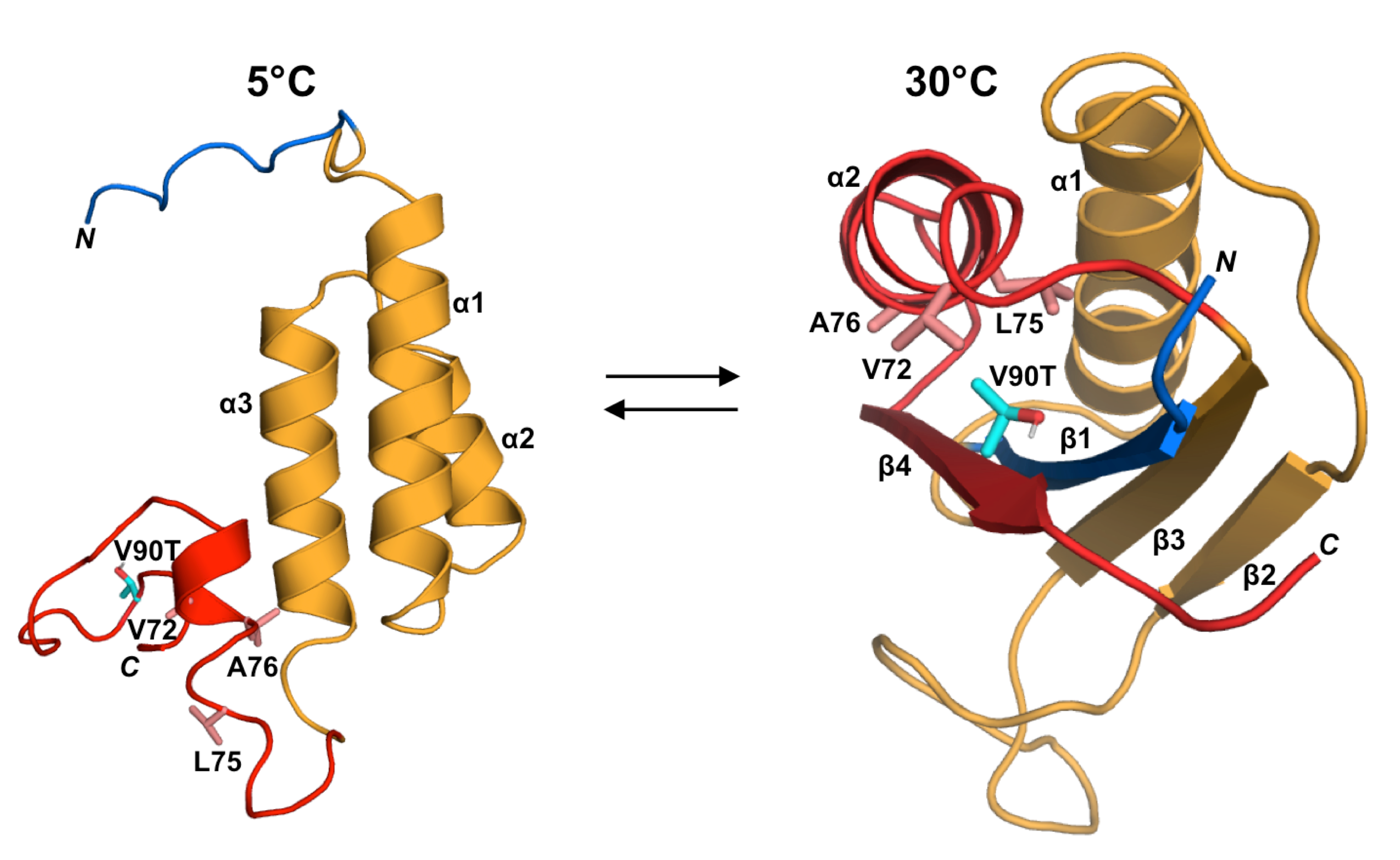
Recently, there have been significant advances into understanding the protein folding code - that is, how the amino acid sequence of a protein relates to its three-dimensional structure. However, it is known that some naturally occurring proteins can adopt two very different structural states depending on their environment. Such “metamorphic” proteins play important roles in both native biological processes and disease states through their shape-shifting properties. Predicting and designing these types of proteins has remained very challenging, suggesting that the folding code is still not fully understood. Two recent papers from the laboratories of John Orban, IBBR Fellow and Professor in the UMD Department of Chemistry and Biochemistry, and Phil Bryan, IBBR Professor Emeritus and Founder of Potomac Affinity Proteins LLC, describe the design and characterization of novel shape-shifting between three of the most common protein folds known. The proteins were designed by embedding the sequence for a small fold within that of a larger structure, creating a critical state in which the latent topology can be exposed with small changes in the amino acid sequence. In some cases, it was possible to shift the equilibrium from one ordered state to a completely different ordered structure without any changes to the amino acid sequence at all. This could be achieved either by employing temperature shifts over a biologically relevant range or by the addition of a protein that acts as a binding partner for one of the states. Dr. Orban comments that the research “at a fundamental level provides insights into how proteins can become shape-shifters, but also could be potentially applied to the design of novel therapeutics that can be turned on and off with environmentally sensitive protein switches”. The papers1,2 appear in January 2023 editions of the journals PNAS and Nature Communications.
- Solomon, T. et al. (2023). Reversible switching between two common protein folds in a designed system using only temperature. PNAS 120, e2215418120. https://www.pnas.org/doi/10.1073/pnas.2215418120
- Ruan, B. et al. (2023). Design and characterization of a protein fold switching network. Nature Communications 14, 431. https://doi.org/10.1038/s41467-023-36065-3
