Patent Pending for Novel Antimicrobial-Development Technology Stemming from IBBR Collaboration
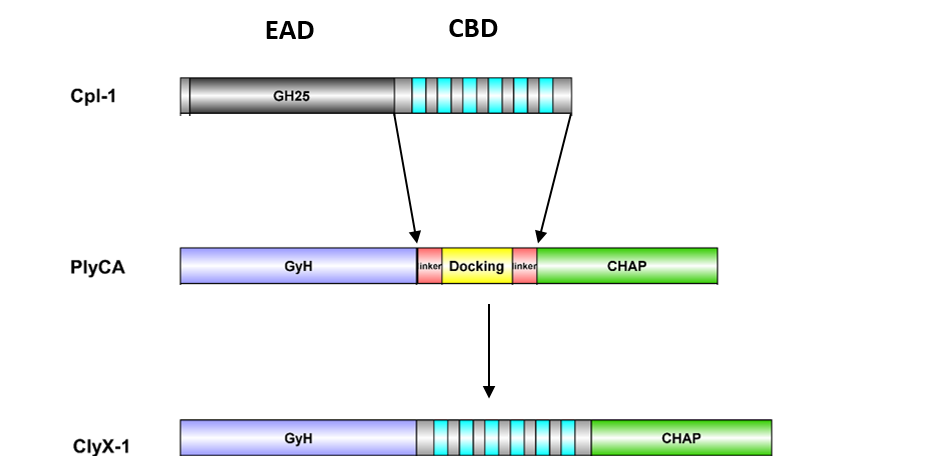
January 22, 2021 - A group of NIST and University of Maryland scientists has applied engineering biology tools to improve and change the properties of a group of bacteriophage proteins called endolysins. A non-provisional patent application entitled “Di-enzymatic chimeric endolysin” with co-inventors IBBR Fellows Zvi Kelman and Daniel Nelson and University of Maryland College Park researcher Xiaoran Shang that describes the technology developed in these studies was submitted to the US patent office and is currently pending.
According to the CDC website, “Antibiotic resistance is one of the biggest public health challenges of our time. Each year in the U.S., at least 2.8 million people get an antibiotic-resistant infection, and more than 35,000 people die.” The development of new frontline antibiotics therefore represents a critical need in healthcare delivery.
The research team works with components of bacteriophages, viruses that infect bacteria, to develop this new technology. Bacteriophages are one of the most abundant self-replicating entities known. Most bacteriophages are very specific for a given bacterial species and are the natural killers of these bacteria. Bacteriophages act as bactericides through endolysins (lysins) that are protein enzymes that degrade the peptidoglycan (PG) in the bacterial cell wall, thereby releasing progeny virions.
A key feature of lysins are that these enzymes, if used alone as antimicrobial compounds in the absence of the parental bacteriophage, are much less likely to develop bacterial resistance (avoiding resistance commonly observed upon treatment with classical antibiotics). Therefore, lysins have gained considerable interest as alternative antimicrobial agents due to their ability to kill Gram-positive pathogens (such as Bacillus anthracis, the etiological cause for anthrax, and Streptococcus pneumoniae, the cause of bacterial pneumonia) effectively when applied externally as a drug.
Endolysins that target Gram-positive bacteria have a modular structure. They have an enzymatically active domain (EAD) and a cell wall-binding domain (CBD). The EAD catalyzes cell wall degradation by cleaving specific bonds in the PG. The CBD is responsible for bacterial specificity. Utilizing the modular nature of these enzyme, Kelman, Nelson, and Shang were able to generate different non-native combinations of EAD and CBD domain constructs with unique bactericidal properties. In one example, the killing efficiency of a pneumococcal endolysin was increased 100-fold by the engineering principles described by the patent. This discovery shows promise for development of new antimicrobial compounds to combat disease-causing bacterial infections.
