-preview.jpg)
Thomas Fuerst
Professor
Fuerst Group (240) 314-6507 tfuerst@umd.eduDr. Fuerst’s research is focused on the development of next-generation vaccines and protein-based therapeutics for infectious disease and cancer. The Fuerst group brings together an assemblage of scientific disciplines including virology, immunology, analytical chemistry, cell biology, structural biology, computational biology, and protein engineering. The multidisciplinary programs include: (1) a structure-based vaccine design program focused on enveloped viruses, (2) a scaffold-based protein therapeutics program focused on cancer targets, and, (3) an immunoadjuvant and delivery program focused on polyphosphazene-based macromolecular delivery systems.
CURRENT RESEARCH
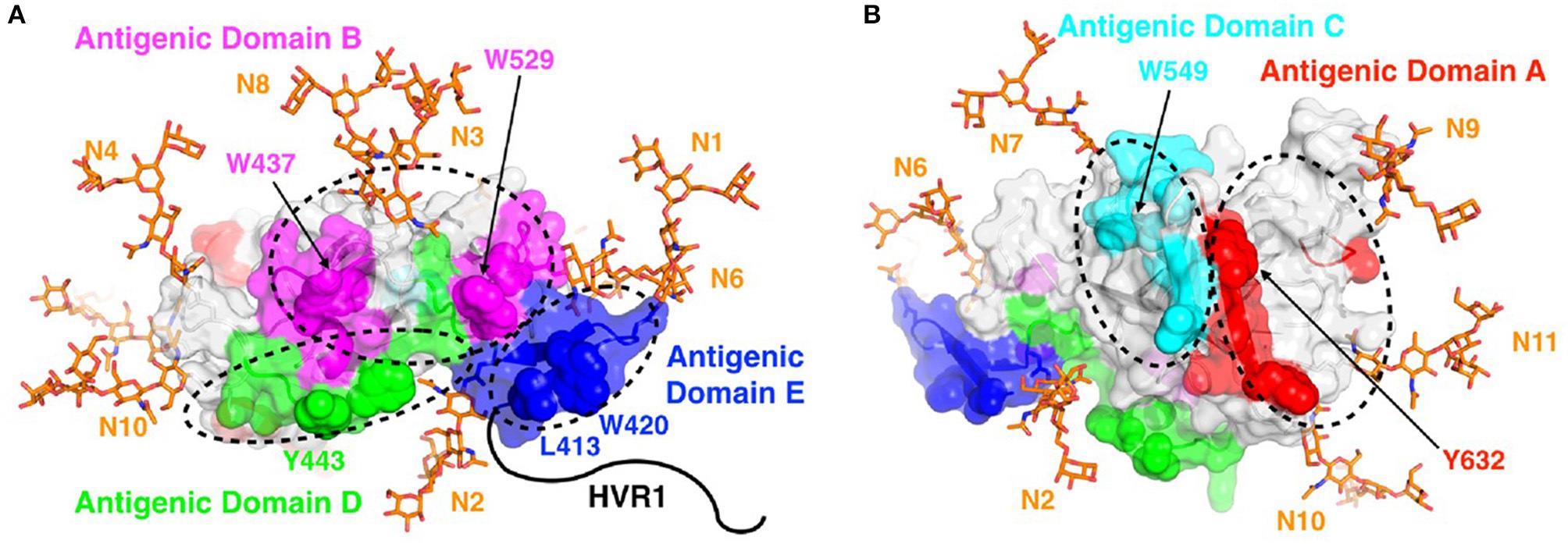
The structure-based vaccine program identifies viral proteins that can be used in vaccines to promote protective immune responses. Hepatitis C virus (HCV), a major human pathogen and a leading cause of liver cirrhosis, liver failure, and hepatocellular carcinoma, is the principal focus of this program. Multiple research studies have suggested that different arms of the immune response are needed for controlling acute and chronic HCV infection. The Fuerst group is defining conserved portions of HCV proteins that can promote protective antibody responses against multiple HCV strains. This novel approach relies on the fundamental principles of structural vaccinology, which involves understanding the nature of neutralizing determinants at the atomic level and applying these insights to develop vaccines that induce protective responses.
drug delivery technologies.
The scaffold-based therapeutic program is developing powerful new classes of protein-based molecules, referred to as SMART molecules, as multi-component protein machines with the potential to undergo changes in conformation in response to binding, which activates a targeted response. SMART molecules can act with low toxicity and have fewer off-target reactions. The group is developing the technology using HRAS, one of the most frequently mutated oncogenes associated with numerous cancers. The SMART molecules under development are expected to sense subtle differences between normal versus oncogenic states in HRAS and compute different therapeutic responses.
The immunoadjuvant and delivery program is customizing a platform for synthesizing multifunctional, biodegradable classes of polymers well-suited for protein stabilization, antigen presentation, and delivery of macromolecules. The research group uses a unique class of polymer called polyphosphazene, which has specialized structural characteristics including a biodegradable backbone. Polyphosphazenes can undergo self-assembly with vaccine antigens and protein therapeutics, and they have unique targeting capabilities, including environmentally-triggered controlled release. Projects within this program focus on immunoadjuvant properties of polyphosphazenes for vaccine delivery and targeted nanoparticle delivery for protein-based therapeutics.
Publications
- The conserved bridging domain on HCV E1E2 glycoprotein complex is targeted by neutralizing antibodies from diverse lineages.
- Polyphosphazene-Mediated Assembly of TLR4 and TLR7/8 Agonists Enables a Potent Nano-Adjuvant Delivery System for Hepatitis C Virus Vaccine Antigens.
- Expression and characterization of SARS-CoV-2 spike protein in Thermothelomyces heterothallica C1.
- Enhanced Production of HCV E1E2 Subunit Vaccine Candidates via Protein-Protein Interaction Identification in Glycoengineered CHO Cells.
- Glycoengineering of the hepatitis C virus E2 glycoprotein improves biochemical properties and enhances immunogenicity.
- Enhanced Production of HCV E1E2 Subunit Vaccine Candidates via Protein-Protein Interaction Identification in Glycoengineered CHO cells.
- Native-like soluble E1E2 glycoprotein heterodimers on self-assembling protein nanoparticles for hepatitis C virus vaccine design.
- Cryo-EM structures of HCV E2 glycoprotein bound to neutralizing and non-neutralizing antibodies determined using bivalent Fabs as fiducial markers.
- Glycoengineering of the hepatitis C virus E2 glycoprotein leads to improved biochemical properties and enhanced immunogenicity.
- Virus-Mimicking Polymer Nanocomplexes Co-Assembling HCV E1E2 and Core Proteins with TLR 7/8 Agonist-Synthesis, Characterization, and In Vivo Activity.
- Macaque antibodies targeting Marburg virus glycoprotein induced by multivalent immunization.
- Hepatitis C Virus E1E2 Structure, Diversity, and Implications for Vaccine Development.
- Glycoengineered recombinant alpha1-antitrypsin results in comparable in vitro and in vivo activities to human plasma-derived protein.
- Site-directed neutralizing antibodies targeting structural sites on SARS-CoV-2 spike protein.
- Prospects for developing an Hepatitis C virus E1E2-based nanoparticle vaccine.
- Structure of engineered hepatitis C virus E1E2 ectodomain in complex with neutralizing antibodies.
- Glycosylation shapes the efficacy and safety of diverse protein, gene and cell therapies.
- Fluorine-Functionalized Polyphosphazene Immunoadjuvant: Synthesis, Solution Behavior and In Vivo Potency.
- Skin Vaccination with Ebola Virus Glycoprotein Using a Polyphosphazene-Based Microneedle Patch Protects Mice against Lethal Challenge.
- An Antigenically Diverse, Representative Panel of Envelope Glycoproteins for Hepatitis C Virus Vaccine Development.
- Structural and Biophysical Characterization of the HCV E1E2 Heterodimer for Vaccine Development.
- Immunopotentiating and Delivery Systems for HCV Vaccines.
- Supramolecular assembly of Toll-like receptor 7/8 agonist into multimeric water-soluble constructs enables superior immune stimulation in vitro and in vivo.
- Engineering subtilisin proteases that specifically degrade active RAS.
- Intracellular Delivery of Active Proteins by Polyphosphazene Polymers.
- Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer.
- Crystal Structure of a Bivalent Antibody Fab Fragment.
- Structure-Based Design of Hepatitis C Virus E2 Glycoprotein Improves Serum Binding and Cross-Neutralization.
- In Vivo and In Vitro Potency of Polyphosphazene Immunoadjuvants with Hepatitis C Virus Antigen and the Role of Their Supramolecular Assembly.
- Protein-loaded soluble and nanoparticulate formulations of ionic polyphosphazenes and their interactions on molecular and cellular levels.
- Antigenicity and Immunogenicity of Differentially Glycosylated Hepatitis C Virus E2 Envelope Proteins Expressed in Mammalian and Insect Cells.
- In vivo combination of human anti-envelope glycoprotein E2 and -Claudin-1 monoclonal antibodies for prevention of hepatitis C virus infection.
- Global mapping of antibody recognition of the hepatitis C virus E2 glycoprotein: Implications for vaccine design.
- Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute hepatitis C virus infection in mice.
- Molecular-Level Interactions of Polyphosphazene Immunoadjuvants and Their Potential Role in Antigen Presentation and Cell Stimulation.
- Self-assembly of polyphosphazene immunoadjuvant with poly(ethylene oxide) enables advanced nanoscale delivery modalities and regulated pH-dependent cellular membrane activity.
