
Vitalii Silin
Dr. Vitalii Silin is a physicist with a wide interdisciplinary background that includes spectroscopy, optics, electrochemistry, neutron reflectometry (NR), electron microscopy (EM), biophysics and biochemistry. He studies the structure and function of integral membrane proteins (IMPs) and peptides imbedded into phospholipid bilayers. Dr. Silin’s laboratory uses a platform developed at NIST and IBBR based on tethered bilayer phospholipid membranes (tBLMs) and synchronized surface plasmon resonance/electrochemical impedance spectroscopy (SPR/EIS) measurements, as well as NR and cryogenic EM, to study membrane active biomolecules.
CURRENT RESEARCH
The study of the structure and function of IMBs is directed related to the design of modern drugs and the development of new therapeutic strategies to fight cancer, neurological disorders, and bacterial and viral infections. The Silin lab investigates membrane receptors and enzymes, toxins and anti-bacterial and anti-viral peptides. In vitro studies of biomolecules inside of membranes present significant challenges, including expression, purification, and reconstitution of IMPs into artificial phospholipid membranes. To overcome these challenges, the lab develops new methods, such as free cell protein expression and enzyme-mediated, two-step assembly of trans-membrane proteins at the surface of the tBLM.
One project in the Silin lab studies the breakup of unsaturated phospholipid membranes by human monoacylglycerol lipase (hMAGL), which plays an important role in the interaction between the cannabinoid receptor CB2 and their ligands, and the role of the binding pocket of hMAGL in this process. The lab is also working on the development of a biochip for the detection of Bacillus anthracis lethal factor and therapeutic agents against anthrax toxins. With the help of neutron reflectometry, the lab identified the structural features and role of the amphipathic domain of tubulin interaction with biomimetic mitochondrial membranes. In collaboration with other scientists, the lab also conducts investigations on antiviral peptides and metal ion-activated pistidins – antimicrobial peptides that have the potential to treat cancer.
Most recently, the Silin lab began working on procedures to form asymmetric, freestanding, phospholipid bilayers (FSPB) that have different compositions of upper and lower leaflets to mimic real cell membranes better. Along with new measurement methods, such as cryogenic EM and the SPR micro-measurement setup, this work will elucidate the biochemical machinery of IMBs.
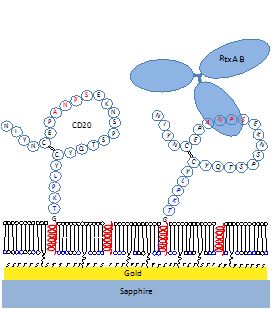
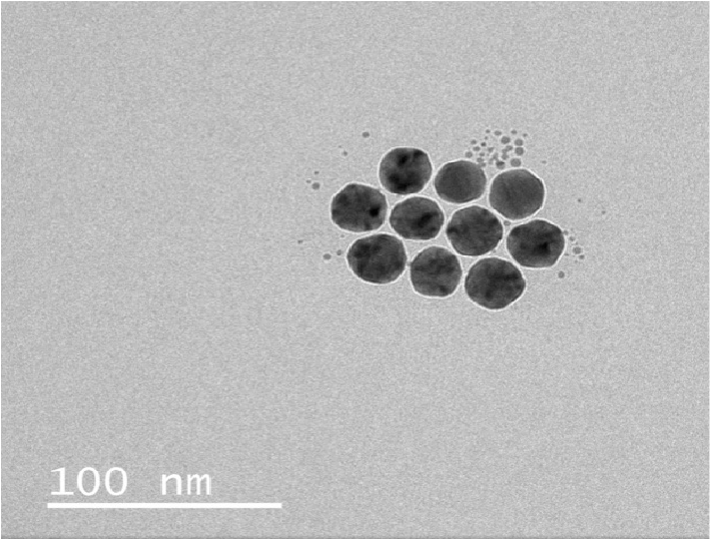
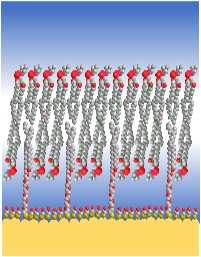
An important part of the Silin lab’s scientific activity is designing and building custom instruments specifically dedicated to the study of IMPs in biomimetic phospholipid membranes.
Publications
- Solution Structures of Bacillus anthracis Protective Antigen Proteins Using Small Angle Neutron Scattering and Protective Antigen 63 Ion Channel Formation Kinetics.
- Copper-binding anticancer peptides from the piscidin family: an expanded mechanism that encompasses physical and chemical bilayer disruption.
- pH dependent electrical properties of the inner- and outer- leaflets of biomimetic cell membranes.
- Development of surface-based assays for transmembrane proteins: selective immobilization of functional CCR5, a G protein-coupled receptor.
- Application of electromodulated fluorescence to the study of the dynamics of single-strand oligonucleotides at modified gold surfaces.
- Isostructural self-assembled monolayers. 2. Methyl 1-(3-mercaptopropyl)-oligo(ethylene oxide)s.
