News Stories
.png)
Daniel Nelson Named Fellow of the National Academy of Inventors
We are proud to congratulate Dr. Daniel Nelson, IBBR Fellow and Professor in the Department of Veterinarian Medicine at University of Maryland, College Park, on being elected a Fellow of the National Academy of Inventors (NAI). This prestigious honor is one of the highest professional distinctions awarded to academic inventors....
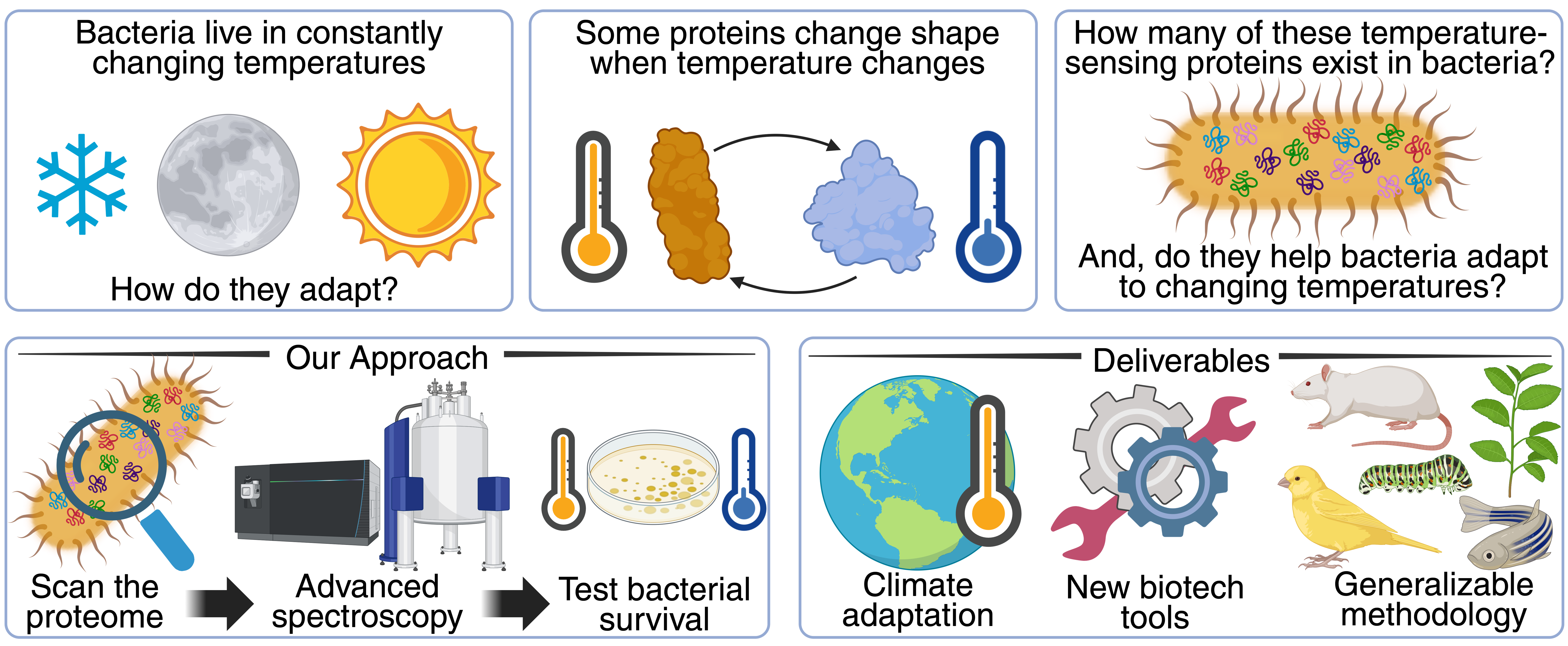
$1.2M Keck Foundation Award Supports IBBR Research on Shape-Shifting Proteins
IBBR is proud to congratulate Dr. John Orban’s lab, along with collaborators from UC-Merced and Caltech, on receiving a $1.2 million research award from the W.M. Keck Foundation. This prestigious grant will propel an ongoing project exploring metamorphic proteins; proteins that can adopt more than one folded state. Unlike endotherms...
.png)
Manghrani Wins Poster Award at the Annual Oligonucleotide Therapeutics Society Meeting
Akanksha Manghrani, a NIST–IBBR postdoctoral research associate in the Biomolecular Measurement Division of the Materials and Measurements Laboratory, received a poster award at the Annual Oligonucleotide Therapeutics Society (OTS) Meeting held in Budapest, Hungary this October. The OTS annual meeting is the premier global conference for researchers and industry leaders...
About IBBR
IBBR is a joint research enterprise of the University of Maryland, College Park, the University of Maryland, Baltimore, and the National Institute of Standards and Technology.
IBBR leverages state-of-art integrative methods for bioanalytical, biophysical and structural characterization of biomolecules: cryo-electron microscopy, nuclear magnetic resonance, x-ray crystallography, small angle neutron and x-ray scattering and mass spectrometry.
IBBR researchers seek to advance therapeutic development, biomanufacturing, and state-of-the-art measurement technologies, to support accelerated delivery of safe and effective medicines to the public.
IBBR is a major initiative and supported in part by the University of Maryland Strategic Partnership: MPowering the State (MPower) , an initiative designed to achieve innovation and impact through collaboration.
Connecting
IBBR Commons
Sophisticated state-of-the-art instrumentation and facilities, and in-house expertise located in shared space and dedicated to advance research, support collaboration and foster innovation of methods. Instrumentation and facilities include tools for high-resolution structural biology, bioanalytical and biophysical measurement, protein engineering and cell culture, advanced computation including artificial intelligence and deep learning methods, and general laboratory services. These capabilities and advanced training are available to IBBR scientists and collaborators.
IBBR Postdoc Program
The IBBR Postdoc Program (IPP) focuses on collaborative research involving basic science and technology development that advances therapeutic development, vaccine development, and biomanufacturing. IPP Fellow project teams are designed with a combination of the IPP Fellow career goals and priorities of project mentors who can be from academic, government, and/or industrial laboratories throughout the University of Maryland, NIST and the I-270 corridor.
NMRPipe
IBBR is home to NMRPipe, a popular collection of programs and scripts for manipulating multidimensional Nuclear Magnetic Resonance (NMR) data. The use of NMRPipe is noted in roughly 40% of all NMR structures accepted into the Protein Data Bank.

Upcoming Events
Special Seminar: NIST RNA Therapuetics Interest Group (RTIG)
BMD Staff Seminar M. Judge/ K. Anderson, .06/.04
NIST Group Meeting; Ella Mihailescu
Recent Publications
Phenazine-Based Synthetic Biology to Signal Between Cells and Electrodes.
Bioelectronic systems that enable seamless communication between electronic devices and living systems represent a transformative frontier in biotechnology. Among available methodologies, redox...
Computational Ligand-Binding Site Prediction.
Binding site prediction is a critical early step in a ligand discovery project. Computer-aided drug design (CADD) offers a variety of methods to identify putative ligand-binding sites on proteins...
Substrate specificity in a designed RAS-targeting protease is coupled to active site and distal motions.
UNLABELLED
Designing proteases with tailored substrate specificity has emerged as a powerful strategy for manipulating protein function in cells. RAS, a key regulator of cell survival and...
Inference and visualization of complex genotype-phenotype maps.
Understanding how biological sequences give rise to observable traits, that is, how genotype maps to phenotype, is a central goal in biology. Yet our knowledge of genotype-phenotype maps in...
Cryo-EM Structures Reveal the Molecular Basis of Asymmetric Allosteric Activation by MMOB in the Hydroxylase of Soluble Methane Monooxygenase.
Soluble methane monooxygenase (sMMO) catalyzes the hydroxylation of methane at non-heme di-iron active sites under ambient conditions. The regulatory component (MMOB) is essential for catalytic...