New Cell Lines Will Facilitate Optimized Production of Antibody-based Therapeutics
Mon, Aug 13, 2018
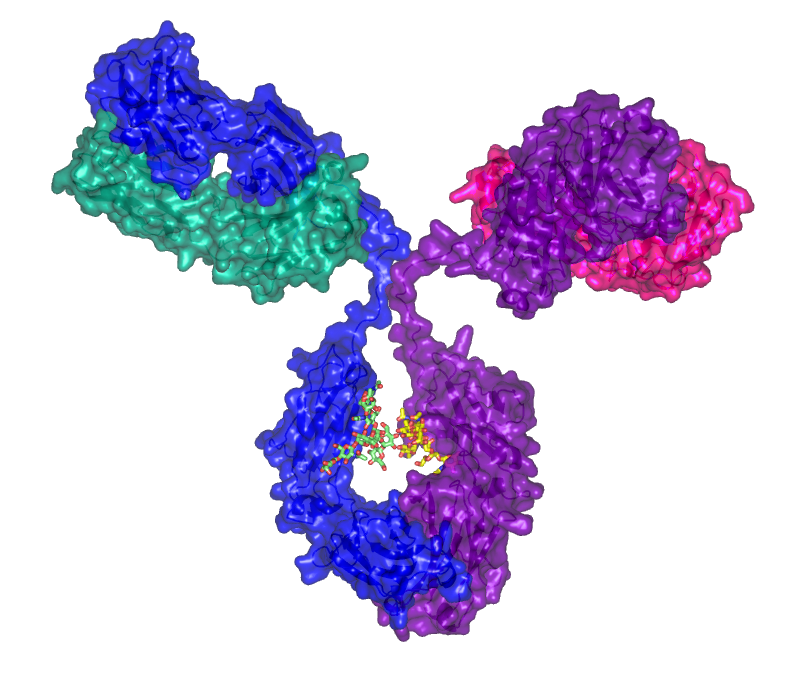
August 13, 2018 -- A family of drugs called monoclonal antibodies (mAbs) harnesses the power of the immune system to fight cancer and autoimmune conditions such as rheumatoid arthritis. Antibodies are large and complicated protein molecules, making optimization, characterization, and regulatory approval a challenge. In 2016, NIST released a reference standard for mAbs called NISTmAb. Now, NIST and UMD researchers at IBBR have developed three cell lines that produce antibodies very similar to the NISTmAb standard. These new cell lines will be publicly available, valuable tools for studying the biomanufacturing production process for new mAb drugs.
Dr. Zvi Kelman led the study team, which included NIST and UMD scientists. This work appears in the journal mAbs.
For additional information, see the news story from NIST here.
-----
Inquiries: communications@ibbr.umd.edu
